Engineering Chemistry Laboratory Practical
1. Preparation of Na2Co3 as a Primary Standard and Estimation of Acidity of a Water Sample Using the Primary Standard
Chemistry (Lab) Practical
(a) To prepare Na2CO3 solution as a primary standard using anhydrous Na2CO3 crystal. (b) To estimate the acidity of given water sample using a standard Na2CO3 solution.
Calculation of Molarity of Na2CO3
Weight
of Salt + bottle (W1) = ……. gm
Weight
of empty bottle (W2) = …...... gm
Weight
of Salt W = (W1 – W2) = …… gm
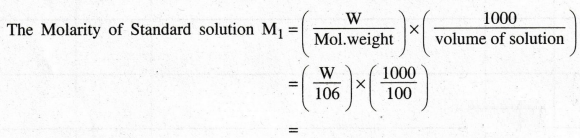
1. PREPARATION OF Na2CO3 AS A PRIMARY
STANDARD AND ESTIMATION OF ACIDITY OF A WATER SAMPLE USING THE PRIMARY STANDARD
Expt.
No.
Date:
AIM
(a)
To prepare Na2CO3 solution as a primary standard using
anhydrous Na2CO3 crystal.
(b)
To estimate the acidity of given water sample using a standard Na2CO3
solution.
MATERIALS
REQUIRED
Burette,
Measuring Jar, Conical flask, Weighing bottle, Std. Flask, Pipette, Burette,
Electronic balance.
CHEMICALS
Na2CO3,
methyl orange and phenolphthalein.
PRINCIPLE:
Acidity
is generally measured by titration with sodium corbonate to an accepted pH
value. Hydrogen ions present, in a sample as a result of dissociation (or)
hydrolysis of solutes, reacts with additions of standard alkali (Na2CO3).
Acidity thus depends on end point of the indicator used. The colour change of
phenolphthalein indicator is close to pH at 25°C corresponds to stoichiometric
neutralization of carbonic acid to bicarbonate.
2H+
+ Na2CO3 → H2O + CO2 + 2 Na+
PROCEDURE:
(A)
Preparation of standard Na2CO3 Solution
Take
approximately 1.06 gm of the Na2CO3 salt in a weighing
bottle and weigh it accurately by using digital balance. Now, transfer the salt
into a 100 ml standard flask through funnel and dissolve the salt with minimum
amount of distilled water. Then make-up the solution up to the mark of the
standard flask and shake well to get uniform concentration. Later, the Molarity
of Na2CO3 solution (M1) can be calculated.
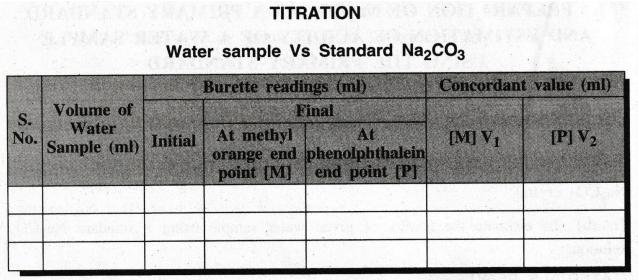
TITRATION
Water Sample Vs. Standard
Calculation
1.
Mineral acidity
Volume
of Na2CO3 (V1) (M) = ........... ml
Normality
of Na2CO3 (N1) = ............... N
Volume
of water sample (V3) = ............... ml
Strength
of water sample (acidity) (N2) = ........
Acidity
(N2) = N1 V1 / V3
Mineral
acidity = …….. N
2.
Total acidity
Volume
of Na2CO3 (V2) (P) = ...... ml
Normality
of Na2CO3 (N1) = ...... N
Volume
of water sample (V3) = …… ml
Strength
of water sample (acidity) (N2) = ........
Total
acidity (N2) = N1 V2 / V3
Total
acidity = ……. N
(B)
Estimation of acidity of water
Burette
is washed with distilled water and rinsed with standard Na2CO3 solution. Then
the burette is filled with the same solution up to the zero mark without any
air bubbles.
20
ml of water sample is pipetted out into a clean conical flask then few drops of
methyl orange indicator is added to the conical flask. The solution is titrated
against Na2CO3 solution till the colour changes from
orange red to yellow. The volume consumed by water sample is noted. Then 2 to 3
drops of phenolphthalein indicator is added to the same solution. The titration
is continued till the colour of water sample is turned to pale pink. The end
point is noted. The titration is repeated until the two concordant values are
obtained.
RESULT:
(i)
Molarity of the Na2CO3 solution =
(ii)
The given sample contains
1.
Mineral acidity =
2.
Total acidity =
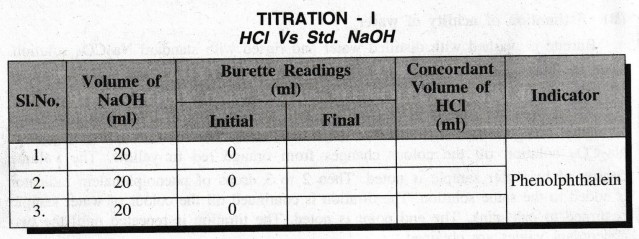
Volume
of NaOH V1 = 20(ml)
Normality
of NaOH N1 = …….. N
Volume
of HCl V2 = ......... ml
Normality
of HCl N2 = ?
According
to the law of volumetric analysis
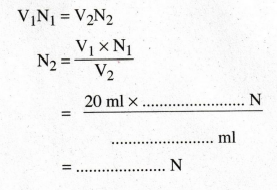
TITRATION
II & III (For Water Sample - 1) Water Sample Vs. Std. HCI
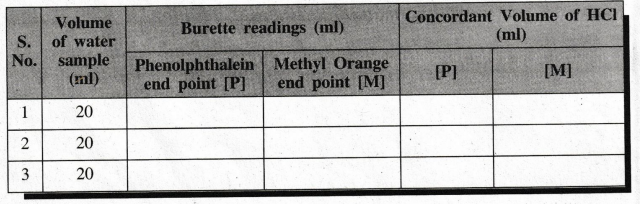
Engineering Chemistry Laboratory Practical : Tag: : Chemistry (Lab) Practical - 1. Preparation of Na2Co3 as a Primary Standard and Estimation of Acidity of a Water Sample Using the Primary Standard
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
