Engineering Chemistry Laboratory Practical
10. Conductometric precipitation titration using BaCl2 - Na2SO4
Chemistry (Lab) Practical
To determine the amount of BaCl2 present in the given solution by conductometric titration using standard Na2SO4 solution of ...... N.
10. CONDUCTOMETRIC PRECIPITATION TITRATION USING BaCl2
- Na2SO4
Expt.
No.
Date:
AIM
To
determine the amount of BaCl2 present in the given solution by conductometric
titration using standard Na2SO4 solution of ...... N.
PRINICIPLE
Solution
of electrolytes conducts electricity due to the presence of ions. Since
specific conductance of a solution is proportional to the concentration of ions
conductance of the solution is measured during titration.
In
the precipitation titration, the ions are converted into insoluble precipitate,
which will not contribute to the conductance. When Na2SO4
is added slowly from the burette to the solution of BaCl2, BaSO4
gets precipitated while the chloride ions remain unchanged.
BaCl2
+ Na2SO4 → BaSO3 ↓ + 2NaCl
The
Ba2+ ions in the solution are replaced by free Na+ ions.
Since the mobility of Nations are less than that of Ba2+ ions, the
conductance of the solution decreases. After the end point, when all the Ba2+
ions are replaced, further addition of Na2SO4 increases
the conductance. This is due to the increase of Na+ and SO2-4
ions in the solution.
MATERIALS
REQUIRED
1.
Conductivity bride 2. Conductivity cell 3. 100 ml beaker 4. Std. Na2SO4
solution 5. Given BaCl2 solution 6. Burette, pipette, glass rod
etc., 7. Distilled water.
PROCEDURE
The
burette is filled with Na2SO4 solution upto the zero
level. 20 ml of the given BaCl2 solution is pipetted out into a clean 100 ml
beaker. The conductivity cell is placed in it and then diluted to 40 ml by
adding conductivity water. The two terminals of the cell are connected with a
conductivity bridge.
Calculation
Volume
of BaCl2 mixture V1
= 20 ml
Strength
of BaCl2 solution N1 = ... ?
Volume
of Na2SO4 V2
= ... ml
Strength
of Na2SO4 N2
= ...... N
According
to law of volumetric analysis V1N1 = V2N2
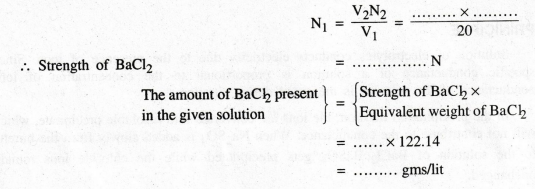
Now
1 ml of Na2SO4 from the burette is added to the solution,
taken in the beaker, stirred and then conductivity is measured. This is
continued up to the end point. (The conductivity is going on decreasing up to
the end point). After the end point, again Na2SO4 is
gradually added and few more readings are noted.
Thus
the conductivity is continuously measured for each addition of Na2SO4
and are tabulated. Now the graph is plotted between the volume of Na2SO4
and conductivity. From the graph, end point is noted and hence the amount of
BaCl2 present in the given solution is calculated.
RESULT
The
amount of BaCl2 present in the given solution = ……… gms.
Engineering Chemistry Laboratory Practical : Tag: : Chemistry (Lab) Practical - 10. Conductometric precipitation titration using BaCl2 - Na2SO4
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
