Engineering Chemistry Laboratory Viva voice questions and answers
11. Estimation of Iron content (Fe2+) by Potentiometric Titration
Chemistry (Lab) Practical : Viva – voice questions & answers
Engineering Chemistry Laboratory : 11. Estimation of Iron content (Fe2+) by Potentiometric Titration : Viva – voice questions & answers
VIVA – VOICE QUESTIONS & ANSWERS
11. Estimation of Iron content (Fe2+) by Potentiometric Titration
1.
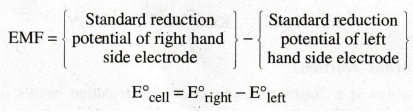
What is emf.
Electromotive
force is defined as, "the difference of potential which causes flow of
current from one electrode of higher potential to the other electrode of lower
potential.
2.
What is the standard solution used in this titration.
K2Cr2O7
3.
What is the name of the instrument used here.
Potentiometer
4.
What is the basic principle of potentiometric titration.
Emf
of a cell depends on the concentration of the electrolytes with which the
electrodes are in contact. Therefore, the electrode reaction is,
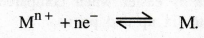
As
the concentration of Mn+ changes, the emf of the cell also changes correspondingly.
5.
Why does emf increase during the titration.
When
it is titrated against the standard K2Cr2O7
solution, taken in the burette, the emf is going on increasing as the
concentration of Fe3+ increases due to the following reaction.
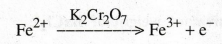
6.
What is the name of the indicator electrode used in this titration.
Platinum
electrode
7.
What is the name of the reference electrode used here, write it representation.
Calomel
electrode; Hg/Hg2Cl2, KCl
8.
Write the cell representation of the titration.o
The
cell is set up by connecting this redox electrode with a calomel electrode as
shown below
Hg
/ Hg2Cl2(s), KC1// Fe2+, Fe3+ / Pt
9.
What is redox reaction.
Redox
reaction is a chemical reactions, where oxidation occurs in one half and
reduction occurs in another part.
10.
Write the nernst equation of a cell.
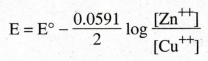
11.
What are the advantages of potentiometric titrations.
(i)
The necessary apparatus required is cheap and easily available.
(ii)
This method can be used for coloured solution.
(iii)
Fixing up end point is easier when compared to the titrations in which indicators
are used to fix up end points.
(iv)
Very dilute solutions can be titrated with accuracy.
(v)
Several components may be titrated in the same solution.
12.
How will you obtain the end point in the titration.
The
accurate end point is determined by plotting ΔE/ΔV Vs Volume of K2Cr2O7
added.
13.
What is the equivalent weight of Fe2+ ion.
55.85
14.
How will you calculate the amount of Fe2+ ions.
Amount
of Fe2+ = 55.85 × N gms/lit
Engineering Chemistry Laboratory Viva voice questions and answers : Tag: : Chemistry (Lab) Practical : Viva – voice questions & answers - 11. Estimation of Iron content (Fe2+) by Potentiometric Titration
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
