Engineering Chemistry Laboratory Practical
12. Estimation of sodium and potassium present in water using flame photometer
Chemistry (Lab) Practical
To estimate the amount of sodium and present in the given water sample using flame photometer.
12. ESTIMATION OF SODIUM AND POTASSIUM PRESENT IN WATER USING
FLAME PHOTOMETER
Expt.
No.
Date:
AIM
To
estimate the amount of sodium and present in the given water sample using flame
photometer.
PRINCIPLE
Flame
photometry (or) Flame Emission Spectroscopy is based on the principle of
emission of certain radiation in the visible region by a metal atom.
In
this technique metal atom is thermally excited from the ground state. The
excited atom emits light of particular intensity while returning to the ground
state depending upon the concentration of the metal ions.
A
water sample to be analysed is sprayed into the flame, where the water
evaporates leaving the fine salt particles. This salt decomposes into
constituent atoms, when they are heated to about 1700°C. The vapours containing
metal atoms are excited by the thermal energy of the flame to higher energy
state.
When
the electrons fall down from the excited state to ground state, they emit
radiation. The emitted radiation is measured and recorded.
The
emission spectrum for each metal is different and its intensity depends on the
concentration of the atoms in the flame.
Sodium
produces a characteristic yellow emission at 589 nm.
Potassium
produces a characteristics red emission at 766 nm.
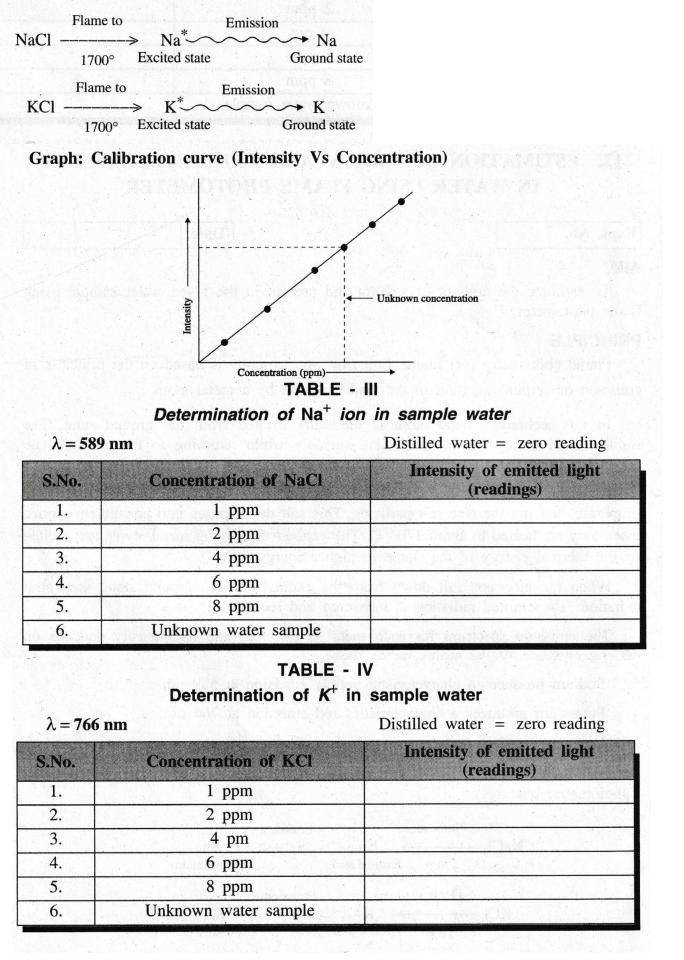
Different standard solutions (say 1, 2, 4, 6, 8 & 10 ppm ) are prepared and the calibration curve is drawn between concentration (ppm) Vs intensity of emitted light (photometric readings).
PROCEDURE
Step
l: Preparation of stock Na+ ion solution
A
stock solution of Na+ ion is prepared by dissolving 25.4 gms of NaCl
in 1 litre of distilled water.
1
ml of stock solution = 10 ppm of Na+ ion
From
the stock solution, various concentrations are prepared (1, 2, 4, 6 and 8 ppm)
as shown in table I.
Step
II: Preparation of stock k solution
A
stock solution of K+ is prepared by dissolving 1.809 gms of KCI in 1
litre of distilled water.
1
ml of stock solution = 1 ppm of K+
From
the stock solution various concentrations are prepared (1, 2, 4, 6 and 8 ppm)
as shown in table II.
Step
III: Working with instrument
The
instrument is switched on. Air supply and gas supply are regulated. First
distilled water is sent and ignition is started. After the instrument is warmed
up for 10 min, the instrument is adjusted for zero reading in the display.
(a) Determination of sodium in the water
sample.
NaCl
solution of 8 ppm is sent and the reading is adjusted for 100. Now the
instrument is said to be calibrated.
The
solution of NaCl of other known concentrations (1, 2, 4 6 and 8 ppm) is sent
one by one and the reading (intensity of emitted light) is noted. The
calibration graph is drawn between the concentration Vs. intensity of the
emitted light (readings).
Now
the unknown water sample is sent and the intensity of emitted light (reading)
is noted. Then the concentration of sodium in the water sample is determined
from the calibration curve.
(b)
Determination of potassium in the water sample
KCI
solution of 8 ppm is sent and the reading is adjusted for 100. Now the
instrument is said to be calibrated.
The
solution of KCl of other known concentration (1, 2, 4 and 6 ppm) is sent one by
one and the reading is noted as before. The calibration graph is drawn between
the concentration Vs readings.
Now
unknown water sample is sent and the reading is noted. Then the concentration
of potassium in the water sample is determined from the calibration curve.
RESULT
(a)
The amount of sodium present in the water sample = …….. ppm
(b)
The amount of potassium present in the water sample = …….. ppm

Engineering Chemistry Laboratory Practical : Tag: : Chemistry (Lab) Practical - 12. Estimation of sodium and potassium present in water using flame photometer
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
