Engineering Chemistry Laboratory Practical
13. Preparation of ZnO nanoparticles by sol-gel method
Chemistry (Lab) Practical
Synthesis of ZnO nanoparticles using ZnCl2, Zn(NO3) 2 and NaOH as precursors.
13. PREPARATION OF ZnO NANOPARTICLES BY Sol-Gel METHOD
Expt.
No.
Date:
AIM
Synthesis
of ZnO nanoparticles using ZnCl2, Zn(NO3) 2
and NaOH as precursors.
MATERIALS
REQUIRED
Zinc
chloride, zinc nitrate, sodium hydroxide and distilled water.
PRINCIPLE
The
basic principle of sol-gel process involves production of solid materials from
small molecules. It involves conversion of monomers into a colloidal solution,
that acts as the precursor. The colloidal solution gradually evolves, towards
the formation of solid ZnO nanoparticles.
PROCEDURE
100
ml of 1.0 M sodium hydroxide solution is taken in a 500 ml beaker and kept in a
magnetic stirrer. It is then heated to about 50° –90°C under constant stirring.
After
obtaining the desired temperature, 100 ml of a solution of 0.5 M zinc chloride
solution and 100 ml of a solution of 0.5 M zinc nitrate solution were slowly
added (dripping) to the reaction mixture. These additions were done one by one
for the period of 50 min. This process was done under constant stirring and the
reaction temperature was maintained at the desired value.
After
dripping, stirring is continued for a period of two hours, maintaining the
desired temperature. The ZnO precipitate formed in the reactor was filtered,
washed and dried in a vacuum oven at 70°C for several hours and weighed.
RESULT
ZnO
nanoparticles were prepared and the yield is = ……… gms.
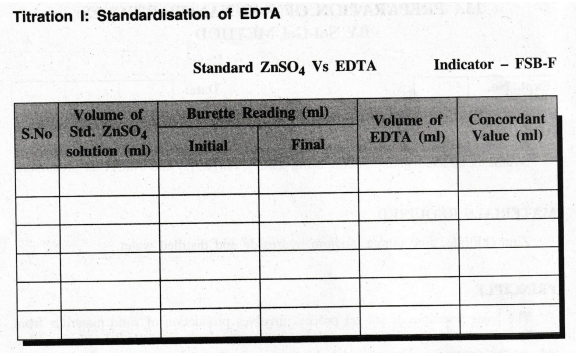
Calculation
Volume
of ZnSO4 V1 = 20
ml
Strength
of ZnSO4 N1 = …..
N
Volume
of EDTA V2 = ...... ml
Strength
of EDTA N2 = ...... ?
According
to the law of volumetric analysis
V1
N1 = V2 N2
N2
= V1 × N1 / V2
Strength
of EDTA N2 = ...... N
Engineering Chemistry Laboratory Practical : Tag: : Chemistry (Lab) Practical - 13. Preparation of ZnO nanoparticles by sol-gel method
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
