Engineering Chemistry Laboratory Practical
15. Proximate analysis of coal
Chemistry (Lab) Practical
To determine moisture, volatile and ash contents in a given coal sample by proximate analysis.
15. PROXIMATE ANALYSIS OF COAL
Expt.
No.
Date:
AIM
To
determine moisture, volatile and ash contents in a given coal sample by
proximate analysis.
PRINCIPLE
Proximate
analysis is a type of analysis of coal. It informs about the practical
utilization of coal. It is the determination of moisture, volatile matter, ash
and fixed carbon. Calorific values mainly depends on the presence of moisture,
volatile matter and ash contents. So it is essential to measure these items.
PROCEDURE
1.
Moisture content
The
given coal sample is air dired. First empty silica crucible is exactly weighed
in an analytical balance, let its weight be X1 gms.
Now
1 gm of air dried coal sample is taken in the silica crucible and let its weight
be X2 gms. The crucible is kept inside the electric oven, maintained
at 110°C, for one hour. After heating, the crucible is removed and kept in a
desiccator for cooling and weighed. Let the weight of the crucible after
cooling be X3 gms.
The
difference in weight is reported in percentage as the amount of moisture in
coal.
2.
Volatile content
The
coal sample, after the determination of moisture content, is heated in a
furnace at 925°C for 7 minutes. After heating, the crucible is removed from the
furnace and cooled in a desiccator. After cooling, it is weighed. Let the
weight of crucible be X4 gms.
Loss
in weight is reported as volatile matter on percentage basis.
3.
% Ash content
Weight
of empty crucible = X1 = ….. gms
Weight
of crucible + sample = X2 = ………. gms

4.
Fixed carbon
%
F.C = 100 – (% moisture + % volatile matter + % Ash)
=
100 – (......... + ......... + .........)
=
............... %
3.
Ash content
The
residual coal, after the analysis of moisture and volatile contents, is heated
in a muffle furnace maintained of 725 – 750°C for 1/2 hour. Heating is done
without crucible lid. The crucible is cooled first in air and then in
desiccator. The crucible is then weighed, let the weight of the crucible be X5
gms.
4.
Fixed carbon content
The
sum total of percentages of moisture, volatile matter and ash, subtracted from
100, gives the percentage of fixed carbon.
RESULT
The
coal sample contains
1.
Moisture = …….. %
2.
Volatile content = ………. %
3.
Ash content = ………. %
4.
Fixed carbon content = ………. %
WEIGHING
AND PREPARATION OF SOME IMPORTANT REAGENTS
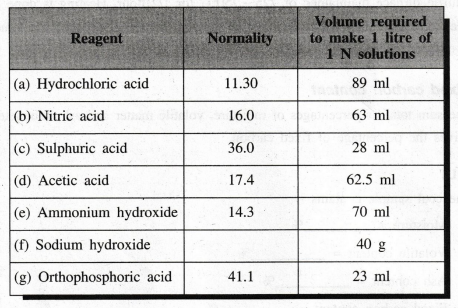
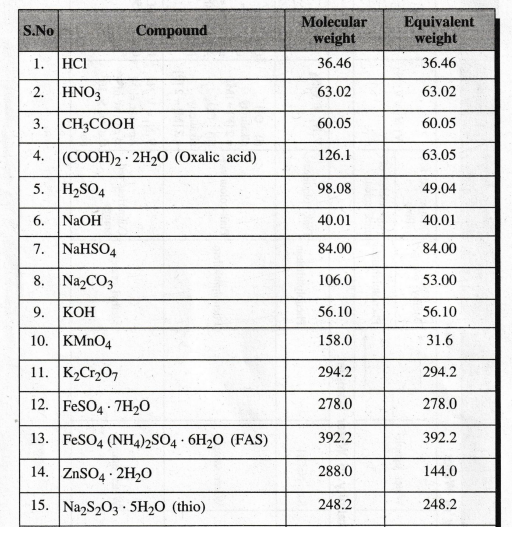
TABLE
OF EQUIVALENT WEIGHTS
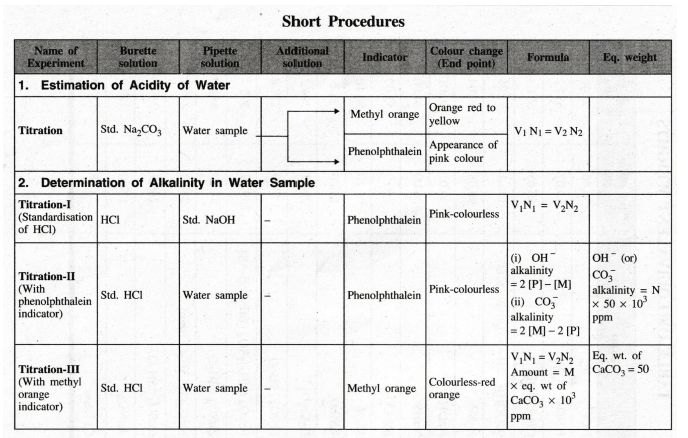
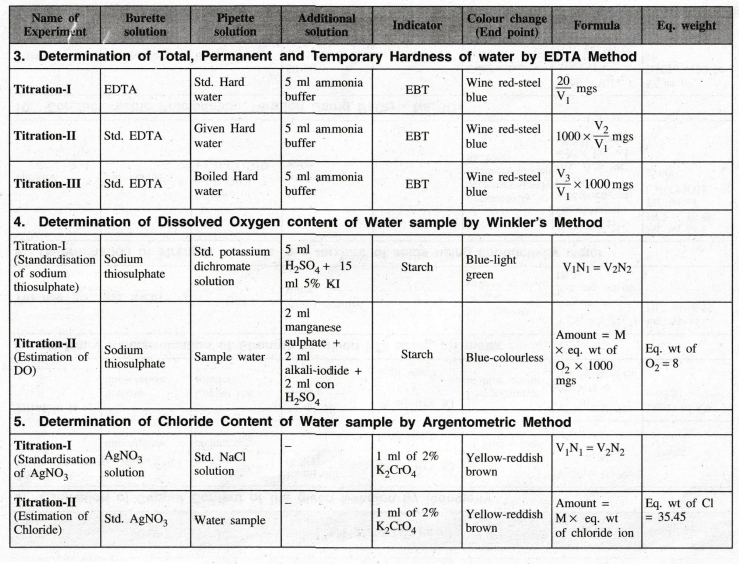
Engineering Chemistry Laboratory Practical : Tag: : Chemistry (Lab) Practical - 15. Proximate analysis of coal
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
