Engineering Chemistry Laboratory Practical
2. Determination of Types and Amount of Alkalinity in Water Sample
Chemistry (Lab) Practical
To determine the type and amount of alkalinity present in the given water sample. A standard solution of sodium hydroxide of strength ............ N and a link solution of hydrochloric acid are provided.
2. DETERMINATION OF TYPES AND AMOUNT OF ALKALINITY IN WATER
SAMPLE
AIM
To
determine the type and amount of alkalinity present in the given water sample.
A standard solution of sodium hydroxide of strength ............ N and a link
solution of hydrochloric acid are provided.
PRINCIPLE
Alkalinity
is caused by the presence of hydroxide, carbonate and bicarbonate. There are
five alkalinity conditions possible in a given sample of water, hydroxide only,
carbonate only, bicarbonate only, combination of carbonate and hydroxide or
carbonate and bicarbonate. The various alkalinities can be determined by
titrating with a standard acid using phenolphthalein and methyl orange
indicators successively.
1.
Phenolphthalein end point
When
alkaline water is titrated with acid using phenolphthalein indicator, hydroxide
alkalinity is completely neutralised and carbonate alkalinity is partially
neutralised.
OH-
+ H+ → H2O
CO2-3+
H+ → HCO-3
2.
Methyl Orange end point
After
the phenolphthalein end point, methyl orange indicator is added and titrated
with acid. Bicarbonate neutralisation occurs.
HCO-3
+ H+ → CO2 + H2O
From
the two titre values the different alkalinities are calculated.
When,
P
= M, → hydroxide alkalinity
2P
= M, → carbonate alkalinity
P
= 0, → bicarbonate alkalinity
Calculations
I.
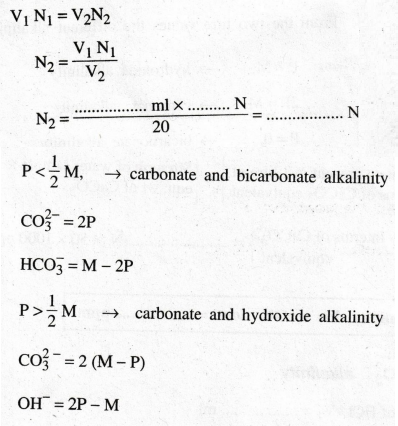
If the data satisfies the condition P > 1/2 M
(i)
Volume of Hcl required for [OH-] alkalinity = 2[P] - [M]
=
2 × ……… - ………
=
……….. ml
(ii)
Volume of HCl required for [CO2-3) alkalinity = 2[M] - 2
[P]
=
2 × ……… - 2× ………
=
……….. ml
(iii)
HCO3- is not present.
Note :
1000
cc of lN HCl = 1 gm equivalent of CaCO3
1000
cc of lN HCl = 50 gm of CaCO3
1.
Calculation of OH- alkalinity
Volume
of HCl V1 = ……….. ml
Strength
of HCl N1 = ……….. N
Volume
of water sample V2 = 20 ml
Strength
of water sample (OH- alkalinity) N2 = ?
According
to the law of volumetric analysis
Alkalinity
values are expressed in terms of milligrams per litre as calcium carbonate.
TABLE
- 1
Titre
values and different alkalinities

i.e.,
OH alkalinity interms of CaCO3 equivalent = …….. N × 50 × 1000ppm
Alkalinity
due to OH- ions = ............. ppm.
2.
Calculation of CO2-3 alkalinity
Volume
of HCl V1 = .................. ml
Strength
of HCl N1 = .................. N
Volume
of water sample V2 = 20 ml
Strength
of water sample (CO2-3 alkalinity) } N2
= ?
According
to the law of volumetric analysis
Alkalinity
due to CO2-3 ions = ............. ppm.
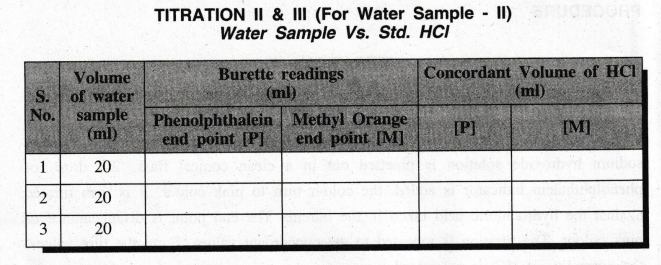
PROCEDURE
TITRATION
– I
Standardisation
of HCI
The
burette is washed well with water and rinsed with the given hydrochloric acid
solution. It is then filled with the same upto zero mark. 20 ml of the standard
sodium hydroxide solution is pipetted out in a clean conical flask. 2-3 drops
of phenolphthalein indicator is added, the colour turn to pink colour. It is
then titrated against the hydrochloric acid taken in the burette. The end point
is disappearance of pink colour. The titration is repeated to get concordant
values. From the titre values, the normality of HCl is calculated.
TITRATION
- II
(With
Phenolphthalein indicator)
20
ml of the water sample is pipetted out in a clean conical flask. A drop of
phenolphthalein indicator is added. Pink colour is observed. This solution is
titrated against the standard HCl, already taken in the burette, until pink
colour is disappeared. The end point is noted. This titre value corresponds to
phenolphthalein end point (P).
TITRATION
- III
(With
Methyl orange indicator)
Few
drops of methyl orange indicator is added to the same solution after the
phenolphthalein end point. The titration is continued until the solution
becomes red orange. The total titre value is noted. This titre value
corresponds to methyl orange end point (M). The titration is repeated for
concordant values.
From
the titre values the amount of each alkalinity present in given water sample is
calculated.
Calculations
II.
If the data satisfies the condition P < 1/2 M
(i)
Volume of HCl required for [CO2-3) alkalinity = 2 [P]
=
2 × ………
=
……….. ml
(ii)
Volume of HCl required for [HCO-3 ) alkalinity = [M] –
2[P]
=
…….. – [2 × ............... ]
=
........... ml
1.
Calculation of CO2-3 alkalinity
Volume
of HCl V1 = .................. ml
Strength
of HCl N1 = .......... N
Volume
of water sample V2 = 20 ml
Strength
of water sample (CO2-3 alkalinity) N2 = ?
According
to the law of volumetric analysis
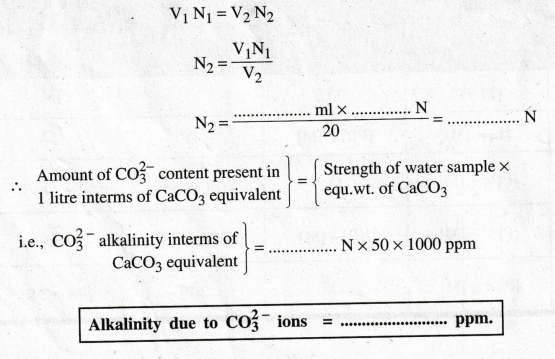
V1N1
= V2N2
N2
= V1N1 / V2

i.e.,
CO2-3 alkalinity
interms of CaCO3 equivalent = …..... N × 50 × 1000 ppm
Alkalinity
due to CO2-3 ions = ........... ppm. |
2.
Calculation of HCO-3 alkalinity
Volume
of HCl V1 = .................. ml
Strength
of HCl N1 = ................ N
Volume
of water sample V2 = 20 ml
Strength
of water sample (HCO-3 alkalinity) } N2 = ?
According
to the law of volumetric analysis

i.e.,
HCO3- alkalinity interms of CaCO3 equivalent =
N × 50 × 1000 ppm
Alkalinity
due to HCO-3 ions = ........ ppm.
RESULTS
I.
Water sample-I contains the following alkalinity
(i)
Hydroxide alkalinity (OH-) = ........ ppm.
(ii)
Carbonate alkalinity (CO23 ) = ……… ppm.
(iii)
Total alkalinity (OH- + CO23) = ..........
ppm.
I.
Water sample-II contains the following alkalinity
(i)
Carbonate alkalinity (CO23 ) = …………. ppm.
(ii)
Bicarbonate alkalinity (HCO3-) = …………. ppm
(iii)
Total alkalinity ((CO23 + HCO3-) = …………. ppm
Structure
of disodium salt of EDTA (Fig.I)
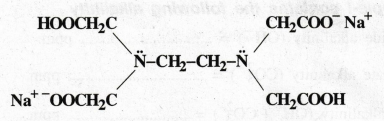
Step
I: STANDARDISATION OF EDTA
TITRATION
– I
Standard
Hard water Vs. EDTA
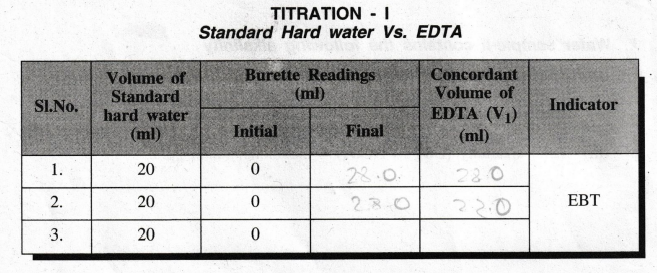
Calculation
Volume
of standard hard water = 20 ml
Volume of EDTA solution consumed, V1 = 28.0 ml
1
ml of standard hard water contains 1 mg of calcium carbonate (CaCO3)
20
ml of standard hard water contains 20 mg of CaCO3
20
ml of standard hard water consumes V1 ml of EDTA
ie.,
V1 ml of EDTA solution = 20 mg of CaCO3
:
1 ml of EDTA solution = 20 / V1 mg of CaCO3 equivalent.
Engineering Chemistry Laboratory Practical : Tag: : Chemistry (Lab) Practical - 2. Determination of Types and Amount of Alkalinity in Water Sample
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
