Engineering Chemistry Laboratory Practical
3 (a) determination of total, temporary and permanent hardness of water by EDTA method (EDTA method - ii)
Chemistry (Lab) Practical
To determine the total hardness, permanent hardness, temporary hardness in the given sample of hard water by EDTA method. A standard hardwater of .............. and a EDTA solution are provided.
3 (a) DETERMINATION OF TOTAL, TEMPORARY AND PERMANENT HARDNESS
OF WATER BY EDTA METHOD
(EDTA Method - II)
Expt.
No.
Date:
AIM
To
determine the total hardness, permanent hardness, temporary hardness in the
given sample of hard water by EDTA method. A standard hardwater of
.............. and a EDTA solution are provided.
PRINCIPLE
Disodium
salt of Ethylene Diamine Tetra Acetic acid (EDTA) is a well known complexing
agent. Its structure is shown in the figure I.
Disodium
salt of EDTA is used to estimate the various hardness of the given hard water
containing Ca2+ and Mg2+ ions. When EDTA is added to hard water, it
reacts with calcium and magnesium ions present in hard water to form stable
EDTA metal complexes. From the volume of EDTA consumed the hardness can be
calculated. Eriochrome Black - T is used as an indicator. The indicator forms a
weak complex with the metal ions present in the hard water and gives wine red
colour.
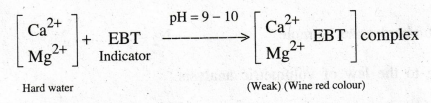
When
EDTA is added into the hard water, the metal ions form a stable metal complex
with EDTA by leaving the indicator. When all the metal ions are taken by EDTA
from the indicator metal ion complex, the wine red colour changes into steel
blue, which denotes the end point. The metal EDTA complex is stable at pH 8-10.
This pH range can be maintained by adding ammoniacal buffer (NH4Cl +
NH4OH).
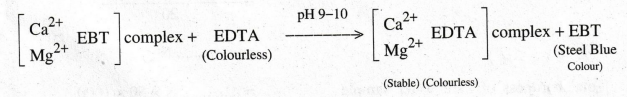
Step
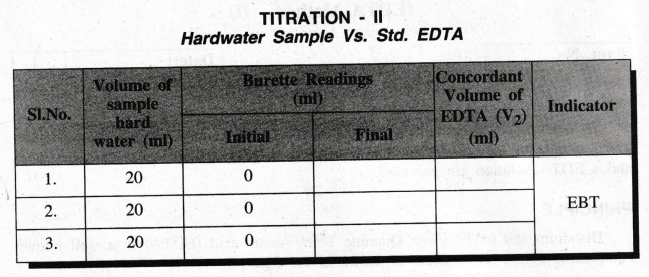
II: DETERMINATION OF TOTAL HARDNESS OF HARDWATER SAMPLE
TITRATION
- II
Hardwater
Sample Vs. Std. EDTA
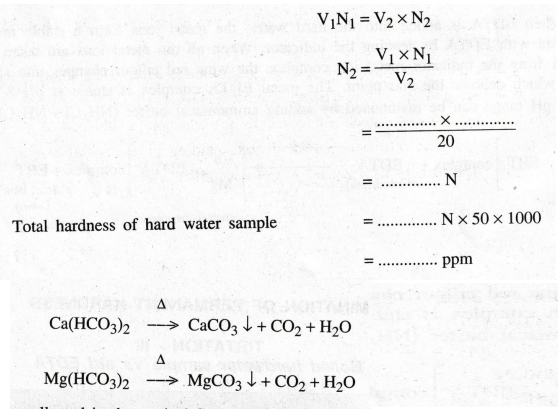
Calculation
of the total hardness of hard water
Volume
of EDTA V1 = ............. ml
Strength
of EDTA N1 = .......... N
Volume
of the hard water sample V2 = 20 ml
Strength
of hard water sample N2 = ............. ?
According
to the law of volumetric analysis
The
filtrate is collected in the conical flask, EBT indicator and buffer solution
are added. It is then titrated against the EDTA.
Temporary
hardness = Total hardness - Permanent hardness
MATERIALS
REQUIRED
1.
EDTA solution 2. Standard hand water 3. EBT indicator 4. Buffer solution 5. Burette,
pipette, conical flask, 250 ml beaker, 100 ml std. flask 6. Sample hard water
PROCEDURE
TITRATION
- 1
Standardisation
of EDTA
Step
I
The
burette is washed well with the distilled water and rinsed with a little amount
of the given EDTA solution. It is then filled with the same EDTA solution upto
the zero level without air bubbles. Initial reading of the burette is noted.
20ml of standard hard water solution is pipetted out into a clean conical
flask. 5ml of ammonia buffer solution and 2 drops of Eriochrome Black - T
indicator are added. The solution turns wine red in colour and it is then
titrated against EDTA taken in the burette. The change of red colour to steel
blue colour is the end point. The final reading in the burette is noted. The
difference in the burette reading gives the volume of the EDTA solution. The
titration is repeated to get concordant values.
Step
III: DETERMINATION OF PERMANENT HARDNESS
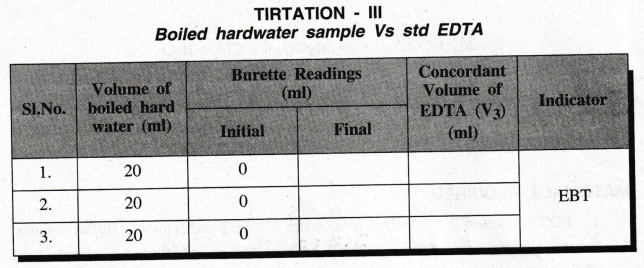
TIRTATION
- III
Boiled
hardwater sample Vs std EDTA
Calculation
of the permanent hardness of the hard water
Volume
of EDTA V1 = .......... ml
Strength
of EDTA N1 = ....... N
Volume
of the boiled hard water sample V2 = 20 ml
Strength
of the boiled hard water sample N2 = ............. ?
According
to the law of volumetric analysis
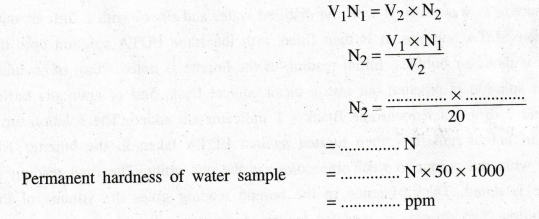
Permanent
hardness of water sample = .............. N × 50 × 1000
=
…..... ppm
Step
IV: Calculation of the temporary hardness of the hard water
Temporary
hardness = Total hardness – Permanent hardness
=
........... - …………..
Step
II
TITRATION
- II
Determination
of Total Hardness of Hardwater Sample
20
ml of the given hardwater sample is pipetted out into a clean conical flask. 5
ml of ammonia buffer solution and 2 drops of Eriochrome Black - T indicator are
added. The solution turns wine red in colour. This solution is titrated against
EDTA solution taken in the burette. The change of wine red colour into steel
blue colour is the end point. The titration is repeated to get concordant
values.
Step
III
TITRATION
- III
Determination
of Permanent Hardness
100
ml of the given sample of hardwater is taken in a clean 250 ml beaker and
boiled for 10-15 minutes. It is then cooled and filtered. The filtrate is
collected in a 100 ml standard flask and made upto the mark using distilled water.
20 ml of this made up solution is pipetted out into a clean conical flask. 5ml
of ammonia buffer solution and 2 drops of Eriochrome Black - T indicator are
added. The solution turns wine red in colour. This solution is titrated against
the EDTA taken in the burette. The change in colour from wine red to steel blue
is the end point. The titration is repeated to get concordant values.
Step
IV
Determination
of Temporary Hardness
Temporary
hardness of the water sample is calculated by subtracting permanent hardness
from total hardness.
Temporary
hardness = Total hardness – Permanent hardness.
RESULT
1.
Amount of total hardness of the given sample of water = ......... ppm.
2.
Amount of permanent hardness of the given sample of water = ……. ppm.
3.
Amount of temporary hardness of the given sample of water = ……. ppm.
Step
I: STANDARDISATION OF SODIUM THIOSULPHATE
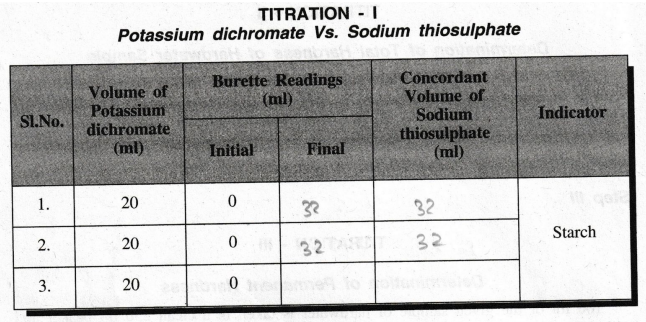
TITRATION
- I
Potassium
dichromate Vs. Sodium thiosulphate
Calculation
Volume
of Potassium dichromate, V1 = 20 ml
Strength of Potassium dichromate, N1 = 0.005 N
Volume of Sodium thiosulphate, V2 = 32.0 ml
Strength
of Sodium thiosulphate, N2 = ?
According
to the law of volumetric analysis
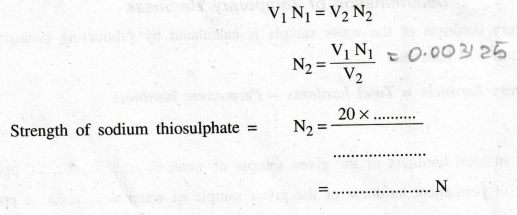
Engineering Chemistry Laboratory Practical : Tag: : Chemistry (Lab) Practical - 3 (a) determination of total, temporary and permanent hardness of water by EDTA method (EDTA method - ii)
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
