Engineering Chemistry Laboratory Viva voice questions and answers
3. Determination of Total, Permanent and Temporary Hardness of Water sample
Chemistry (Lab) Practical : Viva – voice questions & answers
Engineering Chemistry Laboratory : 3. Determination of Total, Permanent and Temporary Hardness of Water sample: Viva – voice questions & answers
VIVA – VOICE QUESTIONS & ANSWERS
3. Determination of Total, Permanent and Temporary Hardness of Water sample
1.
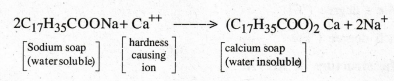
Define hardness of water.
Hardness
is the property (or) characteristics of water, which does not produce lather
with soap.
2.
Define hard water and soft water.
(a)
Hard water
Water,
which does not produce lather with soap solution but produces white precipitate
(scum) is called hard water.
This
is due to the presence of dissolved Ca and Mg. salts.
(b) Soft water
Water,
which produces lather readily with soap solution is called soft water.
This
is due to the absence of Ca and Mg salts.
3.
What are the salts responsible for carbonate and non-carbonate hardness of water.
Carbonate
hardness: Ca(HCO3)2 and Mg(HCO3)
2
Non-Carbonate
hardness: CaCl2, CaSO4, MgCl3,
MgSO4.
4.
How is hardness of water expressed.
The
concentration of hardness producing salts are usually expressed interms of an equivalent
amount of CaCO3.
Significance:
Its molecular weight is whole number and it is the most insoluble salt. If the
concentration of hardness producing salt is X mgs/lit, then
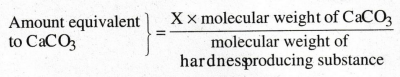
5.
How is temporary hardness removed? Give example.
It
can be removed by (i) boiling the water (ii) adding lime to the water.
The
above two processes convert the bicarbonates into insoluble carbonates and
hydroxides, which can be removed by filtering.
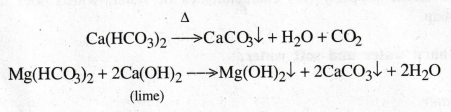
6.
What are the units of hardness.
1.
Parts per million (ppm)
2.
Milligrams per litre (mgs/lit)
3.
Clarke's degree (°C)
4.
French degree (°Fr)
7.
Draw the structure of EDTA.
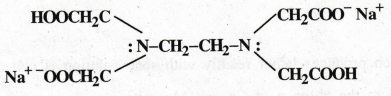
8.
How will you calculate temporary hardness.
Temporary
hardness of the water sample is calculated by subtracting permanent hardness
from total hardness.
Temporary
hardness = Total hardness – Permanent hardness.
9.
What is the colour change when EBT is added to hard water. Write its equation.
The indicator forms a weak complex with the metal ions present in the hard water and gives wine red colour.
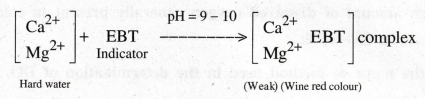
10.
What is a buffer solution.
Buffer
solution is a solution which resists the change in pH by the addition of acid
or base i.e., Buffer solution maintains the constant pH of a solution.
11.
What buffer is used in EDTA titration. Why?
EDTA
make complex with Ca++ & Mg++ ions only at pH 8 - 10.
This range can be maintained by adding ammonia buffer (NH4Cl + NH4OH).
12.
Hardness is expressed in terms of CaCO3
equivalent - comment.
The
concentration of hardness producing salts are usually expressed interms of an
equivalent amount of CaCO3. CaCO3 is chosen as a standard
because,
(i)
Its molecular weight (100) and equivalent weight (50) are whole numbers, so the
calculations in water analysis can be simplified.
(ii)
It is the most insoluble salt, that can be precipitated in water treatment.
13.
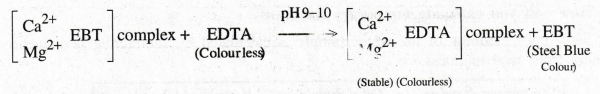
What is the colour change, when EDTA is added to hard water containing EBT. Write
its equation.
When
EDTA is added into the hard water, the metal ions form a stable metal complex
with EDTA by leaving the indicator. When all the metal ions are taken by EDTA
from the indicator metal ion complex, the wine red colour changes into steel
blue.
Engineering Chemistry Laboratory Viva voice questions and answers : Tag: : Chemistry (Lab) Practical : Viva – voice questions & answers - 3. Determination of Total, Permanent and Temporary Hardness of Water sample
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
