Engineering Chemistry Laboratory Practical
4. Determination of dissolved oxygen content of water sample by winkler's method
Chemistry (Lab) Practical
To determine the amount of dissolved oxygen in the given water sample by Winkler's method, a standard solution of K2Cr2O7 of strength .......... N is given.
4. DETERMINATION OF DISSOLVED OXYGEN CONTENT OF WATER SAMPLE BY
WINKLER'S METHOD
Expt.
No.
Date:
AIM
To
determine the amount of dissolved oxygen in the given water sample by Winkler's
method, a standard solution of K2Cr2O7 of
strength .......... N is given.
PRINCIPLE
Oxygen
is dissolved in water to the extent of 7-9 mgs/lit at a temperature range of
25° – 35°C. The estimation of dissolved oxygen in water is useful in studying
corrosion effects of boiler feed water and in studying water pollution. The
amount of dissolved oxygen in water is estimated using Winkler's reagent
(Potassium bromide + Potassium bromate). Water sample is collected carefully
avoiding aeration/deaeration in ground stoppered flask. Initially manganous
sulphate and alkali-iodide reagents are added and the reactions occur as
follows
Mn2+
+ 2OH- → Mn (OH)2 ↓ (White)
Mn(OH)2
+ ½ O2 → MnO(OH)2 + ↓ (Yellow brown)
The
precipitate dissolves in concentrated sulphuric acid liberating iodine and the
liberated iodine is titrated against Na2S2O3.
MnO(OH)
2 + 2H2SO4 →
Mn(SO4) 2 + 3H2O
Mn(SO4)
2 + 2KI → MnSO4 + K2SO4 + I2
2Na2S2O3
+ I2 → Na2S4O6 + 2 Nal
MATERIALS
REQUIRED
1.
Sodium thiosulphate solution 2. Std. Potassium dichromate solution 3. Dil H SO.
4. 5% KI 6. Starch indicator 7. Manganese sulphate 8. Alkali-iodide mixture 9.
Con. H2SO4 10. Burette, pipette, iodine flask.
Step
II : DETERMINATION OF DISSOLVED OXYGEN
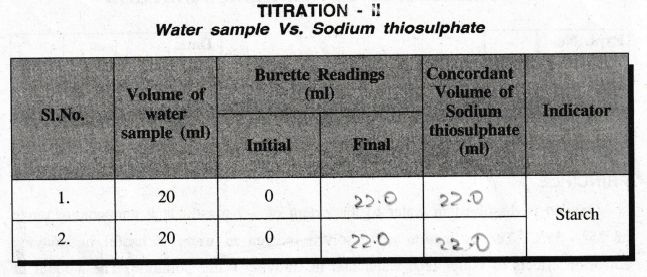
TITRATION
– II
Water
sample Vs. Sodium thiosulphate
Calculation
Volume
of sodium thiosulphate, V1 = 20 ml
Strength
of sodium thiosulphate, N1 = 22.0 N
Volume
of water sample, V2 = 20 ml
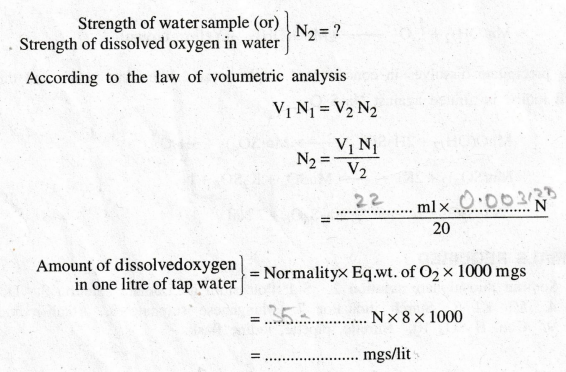
PROCEDURE
TITRATION
- I
Standardisation
of Sodium Thiosulphate
The
burette is washed and rinsed with sodium thiosulphate solution. Then the
burette is filled with the given sodium thiosulphate solution upto zero mark.
20 ml of .......... N potassium dichromate solution is pipetted out into a
clean conical flask. To this, 5 ml of sulphuric acid and 15 ml of 5% potassium
iodide solution are added. This is titrated against sodium thiosulphate
solution. When the solution becomes straw yellow colour, starch indicator is
added and then titration is continued. The end point is disappearance of blue
colour and appearance of light green colour. The titration is repeated to get
concordant value.
TITRATION
– II
Determination
of dissolved oxygen
100-150
ml of the water sample is taken in the iodine flask, 2ml of manganese sulphate
and 2ml of alkali-iodide mixture are added. The stopper is replaced and the
flask is inverted and shaked several times for the rough mixing of reagents.
The flask is left aside for some time. When half of the precipitate settles
down, the stopper is removed and 2 ml of concentrated sulphuric acid is added.
The
stopper is replaced and the flask is inverted several times for complete
dissolution of the precipitate to get a clear yellow solution. 20 ml of this
solution is pipetted out in a clean conical flask and titrated against
standardised sodium thiosulphate solution. When the solution becomes light
yellow, starch indicator is added. The titration is continued until the blue
colour disappears. From the titre value the strength of dissolved oxygen is
calculated and hence the amount of dissolved oxygen in the water sample is
calculated.
RESULT
Amount
of dissolved oxygen in sample water = ………. mgs/lit.
Step
I: STANDARDISATION OF SILVER NITRATE
TITRATION
– I
Standard
NaCl Vs AgNO3
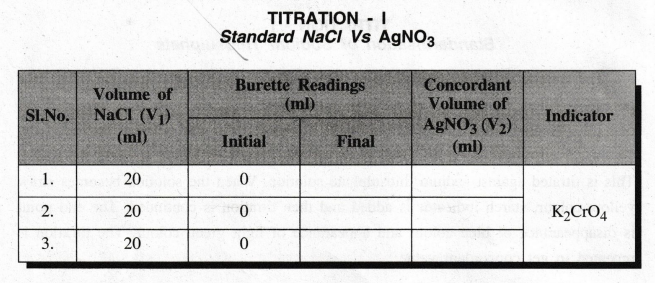
Calculation
of the normality of Silver Nitrate
Volume
of Sodium Chloride V1 = 20 ml
Strength
of Sodium Chloride N1 = ……… N
Volume
of Silver Nitrate V2 = .............. ml
Strength
of Silver Nitrate N2 = ?
According
to the law of volumetric analysis
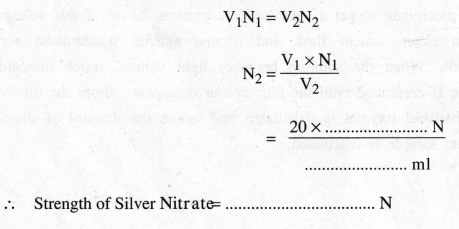
Engineering Chemistry Laboratory Practical : Tag: : Chemistry (Lab) Practical - 4. Determination of dissolved oxygen content of water sample by winkler's method
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
