Engineering Chemistry Laboratory Viva voice questions and answers
5. Determination of Chloride Content in Water by Argentometric Method
Chemistry (Lab) Practical : Viva – voice questions & answers
Engineering Chemistry Laboratory : 5. Determination of Chloride Content in Water by Argentometric Method : Viva – voice questions & answers
VIVA – VOICE QUESTIONS & ANSWERS
5. Determination of Chloride Content in Water by Argentometric Method
1.
What is the form of chloride ions present in water.
Chloride
ions present as salt in water. Example = NaCl, KCI, CaCl2 & MgCl2
2.
What is the name of the method used in this titration.
Mohr's
method
3.
Write the reaction between AgNO3 and chloride ions present in water.
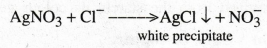
4.
What is the equivalent weight of chloride ion.
35.46.
5.
What is the indicator used in the titration.
Potassium
chromate.
6.
What is the colour change in the titration.
Colour
change form yellow to reddish brown.
7.
Write the reaction between AgNO3 and K2CrO7.
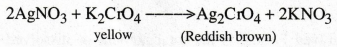
8.
What is the standard solution used in the standardisation of AgNO3.
Standard
NaCl solution.
9.
How to calculate the amount of chloride present in 100 ml of the solution from
the normality of chloride ion solution.
=
35.46 × Normality of Cl- ion × 100 / 1000
Engineering Chemistry Laboratory Viva voice questions and answers : Tag: : Chemistry (Lab) Practical : Viva – voice questions & answers - 5. Determination of Chloride Content in Water by Argentometric Method
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
