Engineering Chemistry Laboratory Practical
5. Determination of chloride content of water sample by argentometric method [mohr's method]
Chemistry (Lab) Practical
To determine the amount of chloride present in 100 ml of the given water sample, being supplied with standard solution of sodium chloride of strength ... N and an approximately N/20 solution of silver nitrate.
5. DETERMINATION OF CHLORIDE CONTENT OF WATER SAMPLE BY
ARGENTOMETRIC METHOD
[Mohr's Method]
Expt.
No.
Date:
AIM
To
determine the amount of chloride present in 100 ml of the given water sample,
being supplied with standard solution of sodium chloride of strength ... N and
an approximately N/20 solution of silver nitrate.
PRINCIPLE
Generally
water contains chloride ions (CI) in the form of NaCl, KCI, CaCl2
and MgCl2. The concentration of chloride ions in water, more than
250 ppm, is not desirable for drinking purpose. The total chloride ions can be
determined by argentometric method (Mohr's Method).
In
this method Cl- ion solution is directly titrated against AgNO3
using potassium chromate (K2C1O4) as an indicator.

At
the end point, when all the ci ions are removed. The yellow colour of chromate
changes into reddish brown due to the following reaction.

MATERIALS
REQUIRED
1.
Std. NaCl solution 2. AgNO3 solution 3. 2% K2CrO4
indicator 4. Burette, pipette, conical flask.
Step
II: DETERMINATION OF CHLORIDE IONS
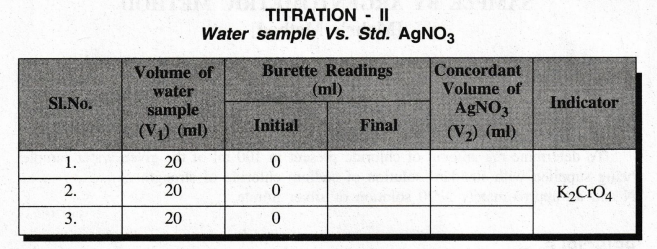
TITRATION
- II
Water
sample Vs. Std. AgNO3
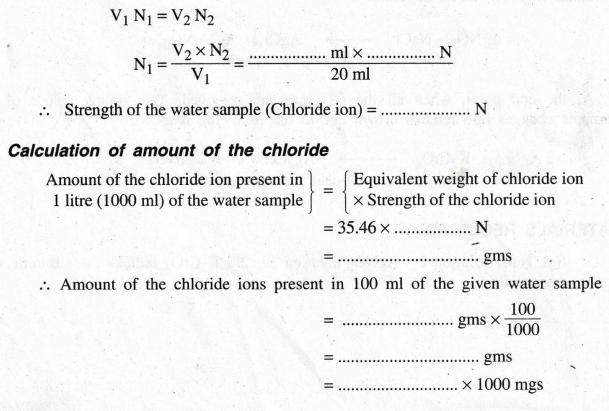
Calculation
of Normality of the water sample (Chloride ions)
Volume
of the water sample V1 = 20 ml
Strength
of the water sample (Chloride ions), N1 = ….. ?
Volume
of silver nitrate, V2 = ................. ml
Strength
of silver nitrate, N2 = …… N
According
to the law of volumetric analysis
PROCEDURE
Step
I
TITRATION
- I
Standardisation
of Silver nitrate
The
burette is washed well with distilled water and rinsed with the small amount of
AgNO3 solution. It is then filled with the same solution upto the
zero mark without any air bubbles.
The
pipette is washed with distilled water and rinsed with the small amount of
standard NaCl solution. 20ml of this solution is pipetted out into a clean
conical flask. 1ml of 2% K2CrO4 indicator solution is
added and titrated against AgNO3 solution taken in the burette. The end point
is the change of colour from yellow to reddish brown. The titration is repeated
for concordant values.
Step
II
TITRATION
II
Determination
of Chloride ions
20
ml of the given water sample is pipetted out into a clean conical flask and 1ml
of 2% K2CrO4 indicator solution is added. It is then titrated
against standardised AgNO3 solution taken in the burette. The end
point is the change of colour from yellow to reddish brown. The titration is
repeated for concordent values.
RESULT
(i)
Amount of chloride ion present in the whole of the given water sample = …….. gms/mgs/ppm
(ii)
Amount of chloride ion present in the 100 ml of the given water sample = ....
gms/mgs/ppm
TITRATION
I: STANDARDISATION OF SODIUM THIOSULPHATE
Standard
potassium dichromate Vs sodium thiosulphate

Volume
of standard potassium dichromate(V1 ) = 20 (ml)
Strength
of standard potassium dichromate (N1) = ...... N
Volume
of sodium thiosulphate solution (V2) = ...... ml
Strength
of sodium thiosulphate solution (N2) = ?
According
to the law of volumetric analysis, V1N1 = V2 N2

Engineering Chemistry Laboratory Practical : Tag: : Chemistry (Lab) Practical - 5. Determination of chloride content of water sample by argentometric method [mohr's method]
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
