Engineering Chemistry Laboratory Practical
6. Estimation of copper content of the given solution by iodometry
Chemistry (Lab) Practical
To estimate the amount of copper present in the given solution by iodometric titration. You are provided with standard ......... N K2Cr2O7 solution.
6. ESTIMATION OF COPPER CONTENT OF THE GIVEN SOLUTION BY
IODOMETRY
Expt.
No.
Date:
AIM
To
estimate the amount of copper present in the given solution by iodometric
titration. You are provided with standard ......... N K2Cr2O7
solution.
PRINCIPLE
Copper
ion occurs naturally in drinking water and is a micronutrient required for the
metabolism of living beings. But the presence of copper in water,in quantities
more than 1.3 mg/l, will cause stomach ache, intestinal distress and digestive
problems. High concentration of copper will also impart a metallic bitter taste
to water. Occurrence of copper ions in drinking water may be due to corrosion
in plumbing materials and faulty water treatment processes. Copper is also
known to cause toxicity to aquatic organism.
Titrimetric
estimation of copper is done through a redox reaction in which stoichiometric
quantity of iodine is liberated on reaction with potassium iodide. The
liberated iodine can be titrated against standardised sodium thiosulphate
solution.
When
KI is added to the copper ion solution, copper ions react with KI and liberates
I2.
2CuSO4
+ 4KI → 2K2SO4 + 2CuI + I2 ↑
The
liberated I2 is titrated against sodium thiosulphate, using starch
indicator.
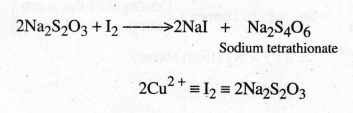
TITRATION
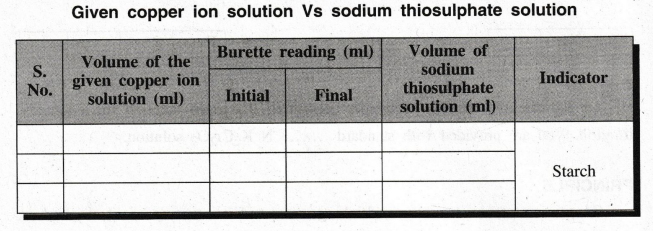
II: ESTIMATION OF COPPER CONTENT OF THE GIVEN
SOLUTION
Given
copper ion solution Vs sodium thiosulphate solution
CALCULATION
Volume
of sodium thiosulphate solution (V1) = ...... ml
Strength
of sodium thiosulphate solution (N1) = ...... N
Volume
of the given copper ion solution (V2) = 20 ml
Strength
of the given copper ion solution (N2) = ?
According
to the law of volumetric analysis, V1 N1 = V2N2
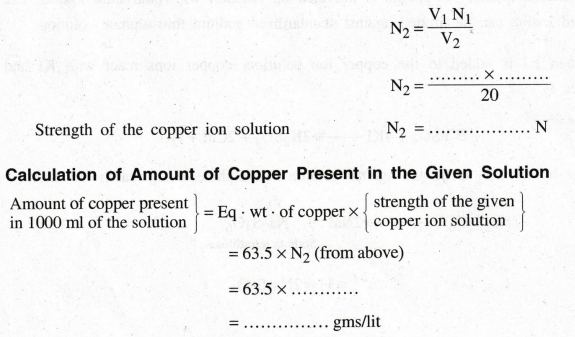
PROCEDURE
TITRATION
I
Standardisation
of sodium thiosulphate
20
ml of standard potassium dichromate solution is pipetted out into a clean
conical flask. About 10 ml of dilute H2SO4 and 10 ml of 10% KI are added to it.
The liberated iodine is immediately titrated against sodium thiosulphate
solution taken in the burette. When the solution turns pale yellow, about 1 ml
of freshly prepared starch is added and the titration is continued. The end
point is the disappearance of blue colour. Titration is repeated for concordant
values.
TITRATION
II
Estimation
of copper ion content of the given solution
20
ml of the given copper ion solution is pipetted out into a clean conical flask.
About 10 ml of dil. H2SO4 and 10 ml of 10% KI solutions
are added to this solution and the liberated iodine is titrated against
standardised sodium thiosulphate taken in the burette. When the solution turns
pale yellow, about 1 ml of freshly prepared starch indicator is added and the
titration is continued. The disappearance of blue colour is the end point. The
titration is repeated for concordant values.
RESULT
1.
Strength of the given copper ion solution = …………. N
2.
The amount of copper in the given copper ion solution = ............ g/lit
TABULATION
Calculation
Weight
of residue w = W2 - W1
=
.................... (-) ...................
=
................... gms
=
× 1000 mgs
=
.................. mgs
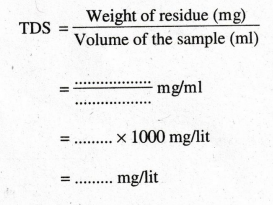
Total
Dissolved Solids (mg/L)
Engineering Chemistry Laboratory Practical : Tag: : Chemistry (Lab) Practical - 6. Estimation of copper content of the given solution by iodometry
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
