Engineering Chemistry Laboratory Practical
8. Determination of strength of given hci using ph meter
Chemistry (Lab) Practical
To determine the strength of given HCl using pH meter, a standard solution of NaOH of .......... N is provided.
8. DETERMINATION OF STRENGTH OF GIVEN HCI USING ph METER
Expt.
No.
Date:
AIM
To
determine the strength of given HCl using pH meter, a standard solution of NaOH
of .......... N is provided.
PRINCIPLE
Since
the pH of the solution is related to the H+ ion concentration by the following formula,
pH
= - log [H]
measurement
of pH of the solution gives the concentration of H+ ions in the
solution. When NaOH is added slowly from the burette to the solution of HCI,
the fast moving H+ ions are progressively replaced by slow moving Na+
ions. As a result pH of the solution increases.
HCI
+ NaOH → NaCl + H2O
The
increase in pH takes place until all the H+ ions are completely
neutralised (upto the end point). After the end point, further addition of NaOH
increases the pH sharply as there is an excess of fast moving OH- ions.
MATERIALS
REQUIRED
(i)
pH meter, (ii) Glass electrode, (iii) 100 Beaker, (iv) Standard NaOH, (v) Given
HCI, (vi) Burette, pipette, Glass rod etc., (vii) Distilled water.
Calculation
Step
I: Calculation of Strength of HCI
Volume
of HCl, V1 = 20 ml
Strength
of the HCl, N1 = ........... ?
Volume
of the NaOH, V2 = ……. (titre value)
Strength
of the NaOH, N2 =.......... N
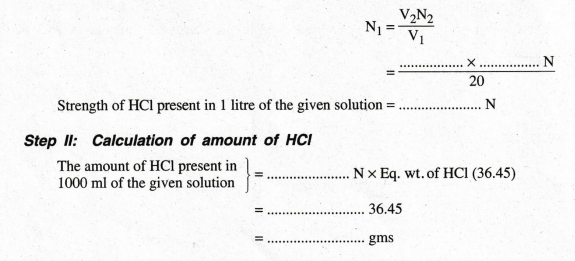
According to the law of volumetric analysis, V1N1 = V2N2
Step
II: Calculation of amount of HCl
The
amount of HCl present in 1000 ml of the given solution = …..... N × Eq. wt. of
HCl (36.45)
=
……….. 36.45
=
……….. gms
PROCEDURE
TITRATION
- I
The
given hydrochloric acid solution is transfered into 100 ml standard flask and
made up to the zero mark using distilled water. 20 ml of this made up solution
is pipetted out into a clean 100 ml beaker and diluted by adding 20 ml of
distilled water The glass electrode is dipped in it and connected with a pH
meter.
The
burette is washed well with water and rinsed with a small amount of given NaOH
solution. It is then filled with the same upto zero mark.
Titration
is carried out by adding std. NaOH solution in portions of 1 ml from the
burette to the HCl solution taken in the beaker and pH of the solution is noted
for each addition. This process is continued until atleast 5 readings are taken
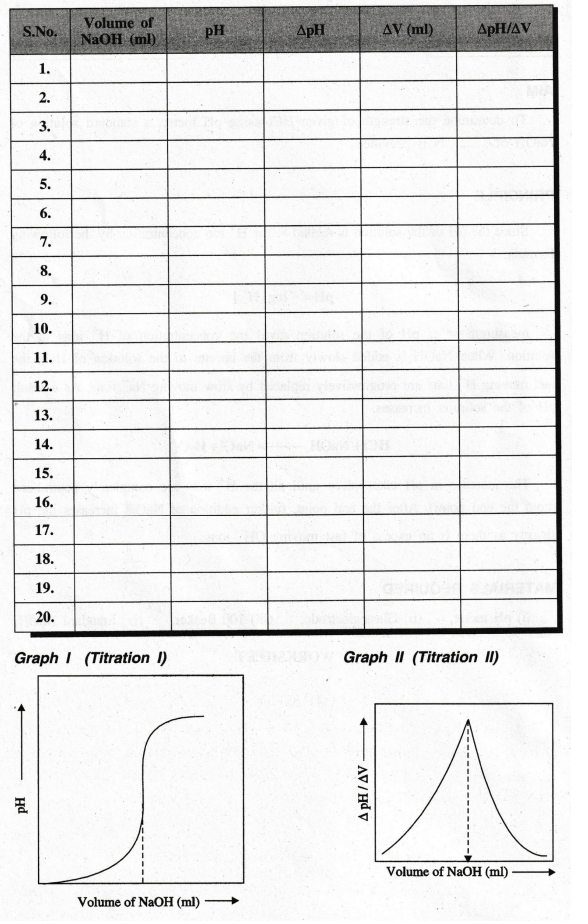
after the end point, and the range at which the end point lies is found out by
plotting volume of NaOH added against pH (graph I).
TITRATION
- II
Another
titration is carried out by adding std. NaOH solution in portions of 0.1 ml
near the end point and pH of the solution is noted after each addition. The
addition of NaOH is continued even after the end point for further 1 ml. The
accurate end point is found out by plotting ΔpH /ΔV against volume of NaOH
added (graph II). From the end point, the strength of HCl solution and hence
the amount of HCl is calculated.
RESULT
1.
Strength of the given HCl solution = …………. N
2.
Amount of HCl present in 1 litre of the solution = …… gms.
Engineering Chemistry Laboratory Practical : Tag: : Chemistry (Lab) Practical - 8. Determination of strength of given hci using ph meter
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
