Engineering Chemistry Laboratory Viva voice questions and answers
8. pH Metry - Determination of Strength of HCI by NaOH
Chemistry (Lab) Practical : Viva – voice questions & answers
Engineering Chemistry Laboratory : 8. pH Metry - Determination of Strength of HCI by NaOH : Viva – voice questions & answers
VIVA – VOICE QUESTIONS & ANSWERS
8. pH Metry - Determination of Strength of HCI by NaOH
1.
What is pH.
pH
is the negative logarithm of H+ ion concentration.
pH
=- log [H+] = log 1 / [H+]
2.
What is pH metry.
Measurement
of pH of a solution using pH meter is called pH metry.
3.
What is the name of the reference electrode used here.
Glass
electrode
4.
How is glass electrode represented.
Pt,
0.1 M HCl/Glass
5.
Write the relation of glass electrode with H+ ion concentration.
Eg
= EGo – 0.0592 log [H+]
(or)
Eg
= EGo + 0.0592 pH
6.
What is the indicator used in this titration.
No
indicator is used
7.
Explain why pH value increases suddenly at the end point.
After
the end point, further addition of NaOH increases pH sharply as there is an
excess of fast moving OH- ions.
8.
What is the pH of a 0.001 M HCI.
pH
= -log [H+]
=
- log [0.001]
=
3
9.
What is the [H] ion concentration if pH of a solution = 5.
pH
= – log [H+]
–
log [H+] = 5
log
[H+] = -5
[H+]
= Antilog [- 5]
Concentration
of H+ = 1 × 10-5M
10.
What is the equivalent weight of HCI.
36.45
11.
How will you calculate the amount of HCI.
Amount
of HCl = 36.45 × N gms/lit
12.
Draw and mention the components of glass electrode.
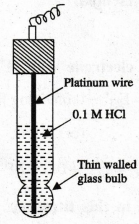
13.
Give an example for ion selective electrode.
Glass
electrode
14.
What is the selectivity of glass electrode.
It
selects only [H+] ions in a mixture.
Engineering Chemistry Laboratory Viva voice questions and answers : Tag: : Chemistry (Lab) Practical : Viva – voice questions & answers - 8. pH Metry - Determination of Strength of HCI by NaOH
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
