Engineering Chemistry Laboratory Viva voice questions and answers
9. Determination of strength of acid in a mixture of acids using conductivity meter
Chemistry (Lab) Practical : Viva – voice questions & answers
Engineering Chemistry Laboratory : 9. Determination of strength of acid in a mixture of acids using conductivity meter : Viva – voice questions & answers
VIVA – VOICE QUESTIONS & ANSWERS
9. Determination of strength of acid in a mixture of acids using conductivity meter
1.
What is weak acid.
If
partial ionisation occurs in a acid, it is a weak acid.
Example:
CH3COOH
2.
What mixture of acids is used in this titration.
HCl
+ CH3COOH
3.
Write the neutralisation reaction between CH3COOH
and NaOH.
CH3
COOH + NaOH → CH3COONa + H2O
4.
Write the strong electrolyte of CH3COOH.
CH3COONa
5.
Why is the conductance increased in the first end point.
I
neutralisation reaction is between HCI & NaOH
HCl
+ NaOH → NaCl + H2O
II
neutralisation reaction is between CH3COOH & NaOH.
CH3COOH
+ NaOH → CH3COONa + H2O
Since
the CH3COOH is partially ionised, only less amount of H+
ions are available to OH- ions. So excess of OH- ions
increases the conductance. Also, CH3COONa is stronger base than the
corresponding acid (CH3COOH).
6.
What are the equivalent weights of HCI & CH3COOH.
HC1
= 36.45
CH3COOH
= 60
7.
Draw the pattern of the graph obtained in this titration.
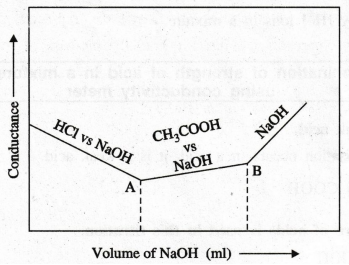
8.
How many end points are obtained from the graph? Comment Two end points.
I
end point is the complete neutralisation of HCl
II
end point is the complete neutralisation of CH3COOH
9.
How will you calculate the amount of HCl and CH3COOH present in a mixture
of acids.
The
amount of HCl = 36.45 × N of HCl gms/lit
The
amount of CH3COOH = 60 × N of CH3COOH gms/lit
10.
What is the solution present in the conductivity cell.
Saturated
potassium chloride (KCI)
Engineering Chemistry Laboratory Viva voice questions and answers : Tag: : Chemistry (Lab) Practical : Viva – voice questions & answers - 9. Determination of strength of acid in a mixture of acids using conductivity meter
Related Topics
Related Subjects
Physics and Chemistry Laboratory
BS3171 Practical Experiment 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
