Environmental Sciences and Sustainability: Unit II: Environmental Pollution
Air Pollution
Definition, Causes, Classification, Common Air Pollutants, Control Measures, Smog
• Air pollution is defined as the undesirable contamination of gas. smoke, dust, fume, mist, odour or chemical particulates in the atmosphere which are injurious to human beings, plants and animals.
Air Pollution
Definition
:
•
Air pollution is defined as the undesirable contamination of gas. smoke, dust,
fume, mist, odour or chemical particulates in the atmosphere which are
injurious to human beings, plants and animals.
Causes
of air pollution
1.
Industrialization
2.
Urbanization
3.
Vehicles emission
4.
Deforestation
5.
Population
1. Classification of Air Pollutants
•
Air pollutants can be broadly classified into two types -
1.
Primary pollutants
2.
Secondary pollutants
1.
Primary pollutants
i)
Pollutants that are emitted directly from either natural events or from human
activities are called primary pollutants.
ii)
The natural events are dust storms, volcano etc and human activities can be.
emission from vehicles, industrial wastes.
iii)
About 90 % of global air pollution is constituted by five primary pollutants.
Examples
i)
Carbon oxides (CO and CO2)
ii)
Nitrogen oxides
iii)
Sulphur oxides
iv)
Hydrocarbons
v)
Particulate matter.
2.
Secondary pollutants
•
Primary pollutants when reacting with each other or from basic components of
air forms a new pollutant called
secondary pollutant.
Examples
:
Sulphuric acid, nitric acid, carbonic acid. etc.
2. Difference between Primary and Secondary Air Pollutants
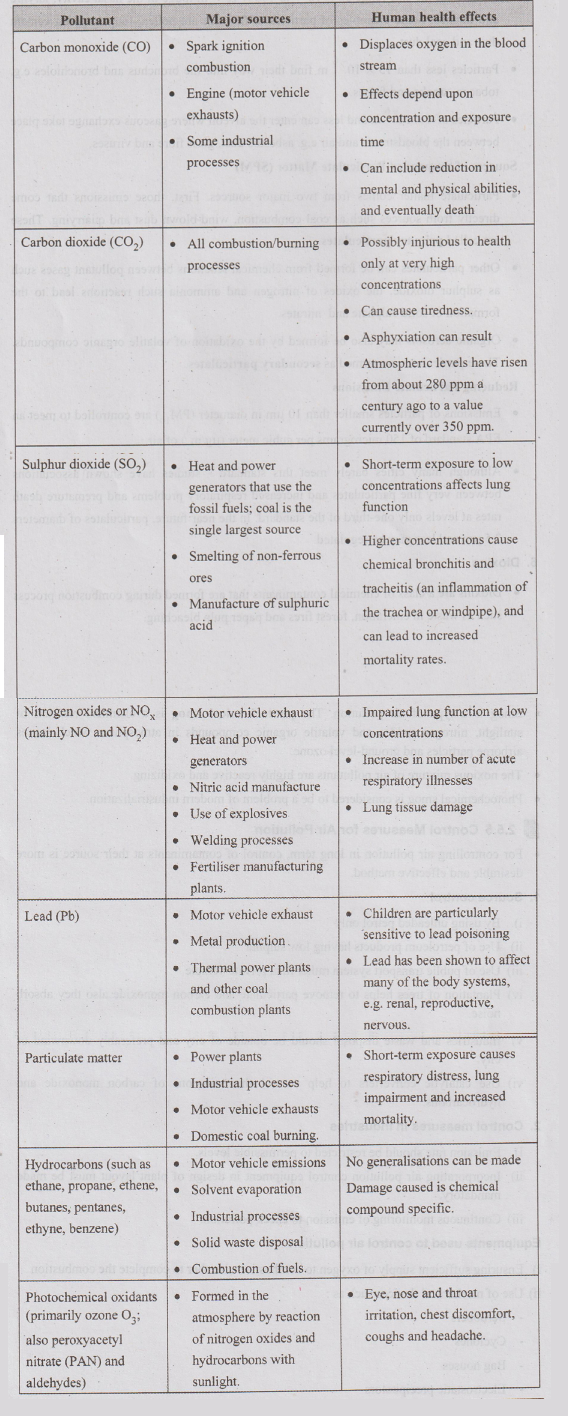
3. Common Air Pollutants
1.
Carbon monoxide (CO)
•
Carbon monoxide (CO) is a colourless odourless, flammable gas, which is a
product of incomplete combustion. If carbon were completely oxidized during
burning, complete combustion to carbon dioxide would occur and carbon monoxide
would not be a problem.
•
It is important not to confuse carbon monoxide with carbon dioxide. Carbon
monoxide (CO) is an incomplete combustion product and can be toxic even at low
concentrations, whereas carbon dioxide (CO2) is a complete oxidation product.
Sources
of carbon monoxide
•
Carbon monoxide is formed whenever a carbon containing material is burned.
•
For example : Automobile exhausts, cigarettes etc. In addition to motor
vehicles, sources of carbon monoxide include burning coal, natural gas or
biomass.
•
Biomass combustion can be a significant source of exposure in rural areas or in
underdeveloped countries where it is burned for cooking, heating and even
light.
•
Atmospheric oxidation of methane gas and other hydrocarbons also produces
carbon monoxide.
Effects
of carbon monoxide
1.
Health effects
•
Many thousands suffer from carbon monoxide-related illness, which include
headaches, dizziness and drowsiness. Reports shows that about 11 % heart
failure caused by excess carbon monoxide.
•
Carbon monoxide also has other adverse effects in the body. For example, it
interferes with the oxygen-carrying proteins in muscles.
•
If the victim continues to receive a high dosage of CO, then permanent brain
damage and even death will result. Initial symptoms include dizziness, headache,
nausea and faintness.
2.
Environmental effects
•
It increases globe temperature.
Measures
to reduce carbon monoxide
•
About half of the motor vehicle carbon monoxide emissions in this country are
produced by only 10 % of the vehicles. Efforts are being made to find and
remove these vehicles from the road.
•
Car and truck owners need to maintain their vehicles so that they operate as
cleanly as they were designed to operate.
•
Other measures to control carbon monoxide emissions include facilities that bum
fossil fuels or wood to maintain high burning efficiencies and prohibiting open
burning of trash and garbage.
2.
Sulphur dioxide (SO2)
•
Sulphur dioxide (SO2) is a colourless gas with a sharp odour that
accounts for about 18 % of all air pollution.
Sources
of sulphur dioxide
1.
Chemical industries
2.
Metal smeltings
3.
Pulp and paper mills
4.
Oil refineries.
Effects
of sulphur dioxide
i)
Health effect
•
Sulphur dioxide reacts with moisture in eyes, lungs and mucous membranes to
form strong irritating acid. It can trigger allergic reaction and asthama.
ii)
Environmental effect
•
Reduced visibility; acid deposition of H2SO4 can damage
trees, soils and aquatic life.
•
The stratospheric ozone depletion, where by sulphate particles in the
stratosphere provide surfaces on which ozone-destroying reactions occur. A
third major effect is the antiwarming influence they exert in global climate
change.
3.
Nitrogen dioxide (NO2)
•
Nitrogen dioxide is a reddish brown irritating gas. They account for about 6 %
of pollution.
Sources
of nitrogen dioxide
1.
Motor vehicle exhausts
2.
Gasoline
3.
Volcanoes
4.
Lightning
Effects
of nitrogen dioxide
i)
Direct, exposure of NO2 irritates eyes and causes infection, asthma.
ii)
Poisonous to plant life. HNO3 can canoed metals and eat away stones.
4.
Lead (Pb)
•
Lead a highly useful metal has been mined for thousands of years. And it has
been known for thousands of years that lead is toxic to the nervous system. The
level of lead in modem human skeletons and teeth is at least a hundred-fold
greater than the level found in pre-industrial age skeletons.
Source
of Lead
•
The combustion of alkyl lead additives in motor fuels accounts for the major
part of all lead emissions into the atmosphere. An estimated 80-90 percent of
lead in ambient air derives from the combustion of leaded petrol.
.
• Paint and storage batteries.
Effects
of Lead
•
Mental retardation, digestion problems, cancer.
•
Harmful to wild life.
5.
Particulate Matter
•
Suspended particulate matter is defined as single particle or aggregates of
particles with diameters greater than 2 × 10-10 m.
•
Some particulate matter is natural i.e. rain. snow. fog. hail and mist, while
others are often the result of human processes, e.g. smoke, soot and fumes.
•
Some natural particulates are affected by human actions such as fog and
wind-blown soils.
•
Smoke and soot are the products of incomplete combustions of coal, petrol and
diesel fuels in furnaces, domestic heating systems and vehicle engines.
Effects
of SPM
•
Aerosols are mixtures of minute solid or liquid particles suspended in air that
form a haze or spoil visibility.
•
The main problem to humans caused by atmospheric particulate matter is how far
it is able to penetrate the respiratory system.
•
Particles in the size range 30 × 10-6 to 100 × 10-6 m
lodge in the nasal cavity, larynre and trachea. Some examples of particles of
this size are pollen, fungal spores, cement dust and coal dust.
•
Particles less than 15 × 10-6 m find their way into the bronchus and
bronchioles e.g. tobacco, smoke and fumes.
•
Particles of 4 × 10-6 m and less can enter the alveoli where gaseous
exchange take place between tile bloodstream and air e.g. asbestos dust, glass
fibre and viruses.
Sources
of Suspended Particulate Matter (SPM)
•
Particulate matter comes from two major sources. First, those emissions that
come directly from sources such as coal combustion, wind-blown dust and
quarrying. These are called primary particulates.
•
Other particulates can be formed from chemical reactions between pollutant gases
such as sulphur dioxide, the oxides of nitrogen and ammonia such reactions lead
to the formation of solid sulphate and nitrates.
•
Organic aerosols may also be formed by the oxidation of volatile organic
compounds. These particulates are termed as secondary particulates.
Reducing
Particulate Emissions
•
Emissions of particles smaller than 10 pm in diameter (PM10) are
controlled to meet an 3 EPA standard of 150 micrograms per cubic meter (µg/m3
) of air.
•
Although many cities barely meet this standard - studies have shown
Associations between very fine particulates and increased respiratory problems
and premature death rates at lex els only one-third of the standard. In the
near future, particulates of diameters 2.5 pm and less may be regulated.
6.
Dioxins
•
Dioxins are a class of chemical contaminants that are formed during combustion
process such as waste in cineration. forest fires and paper pulp bleaching.
Air
Pollutants, Major Source and their Human Health Effect
4. Photochemical Smog
•
Smog is a type of air pollution. The photochemical smog is a chemical reaction
of sunlight, nitrogen oxides and volatile organic compounds in atmosphere which
leaves airborne particles and ground-level ozone.
•
The noxious mixture of air pollutants are highly reactive and oxidizing.
•
Photochemical smog is considered to be a problem of modern industrialization.
5. Control Measures for Air Pollution
•
For controlling air pollution in long term, control of contaminants at their
source is more desirable and effective method.
1.
Source control
i)
By using unleaded petrol only
ii)
Use of petroleum products having low sulphur
iii)
Use of public transport system rather than private vehicle
iv)
Plantation of trees helps to remove particulate and carbon monoxide also they
absorb noise.
v)
Industries and waste disposal should be outside of city and preferably downwind
of city.
vi)
Use catalytic converters to help control the emissions of carbon monoxide and
hydrocarbons.
2.
Control measures in industries
i)
Emission rate should be restricted to permissible levels.
ii)
Incorporating air pollution control equipment in design of plant layout must be
made mandatory.
iii)
Continuous monitoring of emission to check pollution.
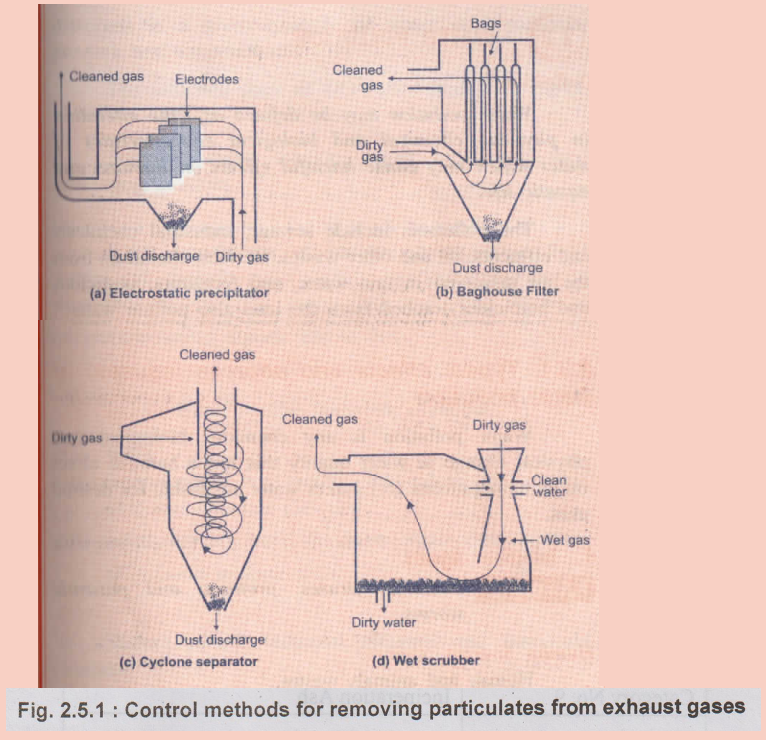
Equipments
used to control air pollution
i)
Ensuring sufficient supply of oxygen to combustion chamber to complete the
combustion
ii)
Use of mechanical devices such as :
-
Scrubbers
-
Cyclones
-
Bag houses
-
Electrostatic precipitators
•
In manufacturing process, electrical power and industrial plants above devices
are used for removing particulates from exhaust gases.
•
All these methods retain hazardous materials of the exhaust which can be
disposed of safely.
•
The set scrubber can be used to remove sulphur dioxide emissions.
Review Questions
1. Define air
pollution. What are the sources of air pollution ?
2. What are the global
impacts of air pollution ?
Environmental Sciences and Sustainability: Unit II: Environmental Pollution : Tag: : Definition, Causes, Classification, Common Air Pollutants, Control Measures, Smog - Air Pollution
Related Topics
Related Subjects
Environmental Sciences and Sustainability
GE3451 ESS 4th Semester | 2021 Regulation | 4th Semester EEE Dept 2021 Regulation
