Engineering Chemistry: Unit I: Water and its Treatment
Anna University 2 Marks Questions and Answers
Water and its Treatment | Engineering Chemistry
Engineering Chemistry :UNIT I : Water and its Treatment : Anna University Two Marks Questions & Answers
Anna University TWO MARKS Questions & Answers
1. Water and its Treatment
1. Define Taste and Odour.
Taste
Taste is the sensation of flavour perceived in the mouth and
throat on contact with a substance.
Odour
Odour is a smell (or) scent caused by one (or) more volatilized
chemical compounds that are generally found in low concentration.
2. What is meant by turbidity ?
Turbidity is the reduction of clarity of natural water due to the
presence of finely divided, insoluble impurities suspended in water.
3. What is the significance of pH in water?
(i) pH determines the solubility (amount that can be dissolved in
water).
(ii) It also determines the biological availability (amount that can
be utilized by aquatic life).
(iii) A rise (or) fall in pH can indicate chemical pollution (or)
acid rain. Many animals cannot live in water at a pH level below 5 (or) above
9.
4. Define hardness.
Hardness is the property (or) characteristics of water, which does
not produce lather with soap.
5. What is BOD.
BOD is defined as, “the amount of free oxygen required by bacteria
for the biological oxidation of the organic matter under aerobic conditions at
20°C for a period of 5 days”.
6. What is the significance of BOD.(APJAKTU, Jan 2016)
(i) It indicates the amount of decomposable organic matter present
in the sewage.
(ii) It enables us to determine the degree of pollution at any time
in the sewage stream.
(iii) Lesser the BOD, better is the quality of water. ie. the water
sample with BOD of less than 3 ppm is considered as pure water, whereas the
water more than 4 ppm is considered as polluted water.
7. Define COD.
COD is defined as, "the measure of amount of oxygen required
to chemically oxidise all the oxidisable impurities present in the sewage using
an oxidising agent like acidified K2Cr2O7":
8. What are the advantages of COD.
(i) Determination of COD is carried out only in 3 hours, but
determination of BOD is carried out after 5 days.
(ii) It measures both the biologically oxidisable and biologically
inert organic matter.
9. What is chlorination.
The process of adding chlorine to water is called chlorination.
Chlorination can be done by the following methods.
(a) By adding chlorine gas
(b) By adding chloramine
(c) By adding bleaching powder
10. What is sterilisation.
The process of destroying the harmful bacterias is known as
sterilisation or disinfection. The chemicals used for this purpose are called
disinfectants.
11. What is break-point chlorination? Explain
Break point chlorination is the point at which all the impurities
are removed and free chlorine begins to appear.
12. What is blow-down operation?
Blow-down operation is a process of removing a portion of
concentrated water by fresh water frequently from the boiler during steam
production.
13. Define desalination.
(Coim AU June 2008) The process of removing common salt (sodium
chloride) from the water is known as desalination. The water containing
dissolved salts with a peculiar salty or brackish taste is called brackish
water.
14. Write the principle involved in the desalination of water by
reverse osmosis. (A.U. (Chem-II) Dec 2006)
(or)
What is meant by 'Reverse osmosis'? How is it applied in the
desalination of water?
(A.U. (Chem-II) June 2007)(CBE AU Jan 2009, Jan. 2018)
If pressure in excess of osmotic pressure is applied on the higher
concentration side, the solvent flow is reversed ie., solvent flows from higher
concentration to lower concentration. This process is called reverse osmosis.
Salt water is taken as higher concentration and water is taken as
solvent. If pressure is applied on the salt water, the water flows from salt
water to water side.
15. Name the method which separate both ionic and non-ionic
impurities from water. [A.U. Nov.96]
Reverse osmosis process.
16. Name some of the membranes employed in reverse osmosis
process. State the advantages of this process. [A.U. Dec.97]
Example: Cellulose acetate, cellulose butrate.
Advantages
(i) It removes ionic as well as non-ionic, colloidal impurities.
(ii) The life time of the membrane is high and it can be replaced
within few minutes.
17. What are the advantages of reverse osmosis method.
(i) The life time of the membrane is high, and it can be replaced
within few minutes.
(ii) It removes ionic as well as non-ionic, colloidal impurities.
(iii) Due to low capital cost, simplicity, low operating, this
process is used for converting sea water into drinking water.
18. Why is water softened before using in boiler? (A.U. June 2007, May 2017)
If hard water obtained from natural sources is fed directly into
the boilers, the following troubles may arise.
1. Scale and sludge formation.
2. Priming and foaming (carry over).
3. Caustic embrittlement.
4. Boiler corrosion.
19. What are scales and sludges? (TNV AU May 2009)
1. Sludge
If the precipitate is loose and slimy it is called sludge. Sludges
are formed by substances like MgCl2, MgCO3, MgSO4
and CaCl2. They have greater solubilities in hot water than cold
water.
2. Scale
On the other hand, if the precipitate forms hard and adherent
coating on the inner walls of the boiler, it is called scale. Scales are formed
by substances like Ca(HCO3)2, CaSO4 and MgCl2.
20. Mention any two disadvantages of formation of deposits in
steam boilers (A.U. Dec 2015)
(or)
What are the disadvantages of scale formation (Chen. A.U. June
2009)
Scales act as thermal insulators. It decreases the efficiency of
boiler. Any crack developed on the scale, leads to explosion.
21. What is meant by priming and foaming? How can they be
prevented. (A.U Oct. 98)
Priming is the process of production of wet steam. Priming can be
prevented by controlling the velocity of steam and keeping the water level
lower.
Foaming is the formation of stable bubbles above the surface of
water. Foaming can be prevented by adding coagulants like sodium aluminate and
antifoaming agents like synthetic polyamides.
22. What is meant by priming and mention its causes (AUT, TNU Jan 2011)
Priming is the process of production of wet steam. Priming is
caused by
(i) High steam velocity.
(ii) Very high water level in the boiler.
(iii) Sudden boiling of water.
(iv) Very poor boiler design.
23. List two disadvantages of using hard water in boilers. (AUT
TNV June 2011, May 2015)
1. Scale and sludge formation
2. Priming and foaming (carry over)
3. Caustic embrittlement
4. Boiler corrosion
24. Mention any two compounds that cause caustic embrittlement in
boilers. (AU June 2014)
Alkali metal carbonates and bicarbonates like Na2CO3,
K2CO3, NaHCO3 , KHCO3
25. What is meant by caustic embrittlement? How is it prevented.
(A.U.June 2007, AU Dec 2009, Jan 2010, Dec 2015, May 2016)
Caustic embrittlement means intercrystalline cracking of boiler
metal.
Prevention
Caustic embrittlement can be prevented by
(i) using sodium phosphate as softening agent instead of sodium
carbonate.
(ii) by adding tannin, lignin to the boiler water, which blocks
the hair cracks.
26. Indicate the reasons for boiler corrosion (Coim. A.U. 2008)
Boiler corrosion arises due to the presence of
(i) dissolved oxygen,
(ii) dissolved carbon dioxide,
(iii) dissolved salts.
27. What are the requisites of drinking and boiler feed water? [A.U. Nov.2001]
(i) Boiler feedwater
1. Must have zero hardness and free from dissolved gases like O2,
CO2.
(ii) Drinking water
1. pH of water should be in the range of 7.0 – 8.5.
2. Total hardness and dissolved solids of water should be less
than 500 ppm.
28. Define softening of water. How is it caried out.
The process of removing hardness producing salts from water is
known as softening (or) conditioning of water.
Softening of water can be done in two methods
1. External treatment. 2. Internal treatment.
29. Soft water is not DM water whereas DM water is soft water -
Justify.
(or)
Distinguish between soft water and demineralised water. (A.U Jan 2013)
The soft water, produced by lime-soda and zeolite processes, does
not contain hardness producing Ca2+ and Mg2+ ions, but it
will contain other ions like Na+, K+, SO2-4,
Cl- etc. On the other hand D.M. (Demineralised) water does not
contain both anions and cations.
30. What are the advantages of ion-exchange process.
(i) Highly acidic or alkaline water can be treated by this
process.
(ii) The water obtained by this process will have very low
hardness (nearly 2 ppm).
31. How is exhausted resin regenerated in ion-exchange process. (Coim A.U. Feb 2010)
When the cation exchange resin is exhausted, it can be regenerated
by passing a solution of dil HCl or dil H2SO4.
RCa + 2HCl → RH2 + CaCl2
RNa + HCl → RH+ NaCl
Similarly, when the anion exchange resin is exhausted, it can be
regenerated by passing a solution of dil NaOH.
R'Cl2 + 2NaOH → R'(OH)2 + 2NaCl
32. Give some examples for cation exchange resin.
(i) Sulphonated coals.
(ii) Sulphonated polystyrene.
33. Give some examples for anion exchange resin.
(i) Cross - linked quaternary ammonium salts.
(ii) Urea - formaldehyde resin.
34. How is boiler corrosion, due to dissolved oxygen, removed.
Sodium sulphite, hydrazine are some of the chemicals used for
removing dissolved oxygen from water.
2Na2SO3 + O2 → 2Na2SO4
N2H4 + O2 → N2 + 2H2O
35. Name the gases dissolved in water that cause corrosion?
(i) Dissolved oxygen (DO)
DO in water attacks the boiler material at higher temperature.
4Fe + 6H2O + 3O2 → 4Fe (OH)3 ↓
(ii) Dissolved carbon dioxide
Dissolved CO2 in water produces carbonic acid, which is
acidic and corrosive in nature.
CO2 + H2O → H2CO3
36. How does carbon dioxide cause boiler corrosion.
Dissolved carbon dioxide in water produces carbonic acid, which is
acidic and corrosive in nature.
CO2 + H2O → H2CO3
Carbon dioxide gas is also produced from the decomposition of
bicarbonate salts present in water.
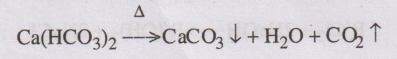
37. What are ion exchange resins? (AUT TNU June 2010)
Ion exchange resins are long chain, cross linked, insoluble
organic polymers with a microporous structure. The functional groups attached
to the chains are responsible for the ion exchanging properties.
38. What are the disadvantages of ion-exchange process.
(i) Water containing turbidity, Fe and Mn cannot be treated,
because turbidity reduces the output and Fe, Mn form stable compound with the
resin.
(ii) The equipment is costly and more expensive chemicals are
needed.
39. How is water demineralised in an ion-exchanger? [A.U Nov.2001] When the
water containing ions (both anion and cation) are passed through
ion exchange columns, it absorbs all the ions (anions and cations) as shown
below.
Cation exchanger: R(H)2 + CaCl2 → RCa + 2HCI
Anion exchanger: R(OH) 2 + 2HCI → RCl2 + 2H2O.
40. What is meant by internal conditioning of water. (A.U May 2014)
Internal conditioning is the process which involves the removal of
scale forming substance by adding chemicals directly into the boiler.
41. Explain the function of a coagulant with example.
When the coagulant is added to water, it gets hydrolysed to form a
gelatinous precipitate of coagulatant Al(OH)3. The gelatinous precipitate,
Al(OH)3, entraps the finely divided and colloidal impurities,
settles to the bottom and can be removed easily.
42. What is phosphate conditioning (Coim A.U. Feb 2010)
(or)
What is the role of phosphates in the internal treatment of water?
(May 2008)
Scale formation can be avoided by adding sodium phosphate. It is
used in high pressure boilers. The phosphate reacts with Ca2+ and Mg2+
salts to give soft sludges of calcium & magnesium phosphates.
3 CaSO4 + 2 Na3PO4 → Ca3(PO4)2
+ 3Na2SO4
43. What are boiler compounds? Mention two different boiler
compounds and their actions. [May 2001,
May 2015]
Scale forming substances can be removed by adding chemicals
directly to the boiler. These chemicals are called boiled compounds.
Examples: Sodium carbonate and
sodium phosphate.
(i) CaSO4 + Na2CO3 → CaCO3 + Na2SO4.
(ii) 3CaSO4 + 2Na3PO4 → Ca3
(PO4)2 + 3Na2SO4.
44. What is calgon conditioning? How is it functioning in water
treatment? (TCY AU July 2008) (Chen
A.U. June 2005, Jan 2010)
(or)
Write the chemical reaction involved in calgon conditioning (AU June 2014)
Calgon is sodim hexa meta phosphate Na2 [Na4 (PO3)6]. This
substance interacts with calcium ions forming a highly soluble complex and thus
prevents the precipation of scale forming salt.
2CaSO4 + Na2 [Na4 (PO3)6]
→ Na2 [Ca2 (PO3)6] + 2Na2SO4
45. Distinguish between internal and external conditioning of
water. (A.U. Dec 2014)
External treatment
1. It is expensive
2. No chemicals are used
3. It is carried out before feeding the water into the boiler.
4. It is used for high pressure boilers.
5. Blow down operation is not required.
Internal treatment
1. It is cheap
2. Chemicals are used
3. It is carried out within the boiler.
4. It is used for low pressure boilers.
5. It requires blow down operation.
46. Why calgon conditioning is better than phosphate conditioning?
(A.U. June 2016)
In calgon conditioning calgon forms highly soluble complex, but in
phosphate conditioning, it gives sludge. So periodical disposal of sludge is
important in phosphate conditioning, but in calgon conditioning no problem of
disposal.
Engineering Chemistry: Unit I: Water and its Treatment : Tag: Engineering Chemistry : Water and its Treatment | Engineering Chemistry - Anna University 2 Marks Questions and Answers
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
