Engineering Chemistry: Unit V: b. Energy Storage Devices
Anna University 2 Marks Questions and Answers
Energy Storage Devices | Engineering Chemistry
Engineering Chemistry : UNIT V : Energy Sources and storage devices : Anna University Two Marks Questions & Answers
8. Batteries
1. What is a battery? How does it differ from a cell? (A.U June 2014, June 2016)
A battery is an arrangement of several electrochemical cells,
connected in series, that can be used as a source of direct electric current.
Thus,
A Cell: Contains only one anode and
cathode.
A Battery: Contains several
anodes and cathodes.
2. What are the important requirement of a battery?
A useful battery should fulfil the following requirements
1. It should be light and compact for easy transport.
2. It should have long life both when it is being used and when it
is not used.
3. The voltage of the battery should not vary appreciably during
its use.
3. What is a primary battery? Give an example (A.U. June 2006)
(or)
What are primary cells?
Primary cells are cells in which the electrode and the electrode
reactions cannot be reversed by passing an external electrical energy. The
reactions occur only once and after use they become dead. Therefore, they are
not chargeable.
Example: Leclanche's cell
4. What are secondary cells? (A.U Jan 2013)
Secondary cells are cells in which the electrode reactions can be
reversed by passing an external electrical energy. Therefore, they can be recharged
by passing electric current and used again and again. These are also called Storage
cells (or) Accumulators.
Example: Lead acid storage cell,
NiCd cell.
5. Will the emf of battery vary with size? Give reason for your
answer. (A.U. June 2014)
No, emf of a battery will not vary with size.
Reason
Emf of a battery depends only on concentration and nature of anode
and cathode.
6. Write the cell representation of lead storage cell.
The cell may be represented as;
Pb / PbSO4 // H2SO4 (aq)
/ PbO2 / Pb
7. State the reaction when a lead storage battery is recharged. (Chen & TNV. A.U. Jan 2009)
The cell can be recharged by passing electric current in the
opposite direction. The electrode reaction gets reversed. As a result, Pb is
deposited on anode and PbO2 on the cathode. The density of H2SO4
also increases.
The net reaction during charging is
8. Write the charging and discharging reaction of lead
accumulator. (Coim AU Jan 2008) charging
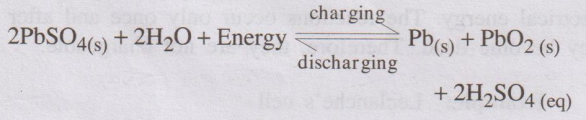
9. What are the applications of lead acid storage cell?
1. Lead storage cell is used to supply current mainly in
automobiles such as cars, buses, trucks, etc.,
2. It is also used in gas engine ignition, telephone exchanges,
hospitals, power stations, etc.,
10. What are the advantages of storage batteries?
(i) It is made easily.
(ii) It produces very high current.
(iii) The self-discharging rate is low.
(iv) It also acts effectively at low temperature.
11. What are fuel cells?
Fuel cell is a voltaic cell, which converts the chemical energy of
the fuels directly into electricity without combustion. It converts the energy
of the fuel directly into electricity. In these cells, the reactants, products
and electrolytes pass through the cell.
Fuel + Oxygen → Oxidation products + Electricity .
12. What are the electrodes used in the fuel cells porous? (CBE. A.U. Jan 2009)
Compressed carbon containing a small amount of catalyst like Pt,
Pd, Ag, are used in the fuel cells porous.
13. What are the applications of H2 -O2 fuel
cell?
1. H2 - O2 fuel cells are used as auxiliary
energy source in space vehicles, submarines or other military-vehicles.
2. In case of H2-02 fuel cells, the product of water is proved to
be a valuable source of fresh water by the astronauts.
14. What are the limitations of H2 - O2 fuel
cell? (A.U. May 2015)
(a) Hydrogen gas is explosive.
(b) It is very expensive to be carried out.
(c) As hydrogen is a gas, it is expensive to compress into
liquid form.
(d) Hydrogen is not present as it is, but always present in combined form with either
oxygen or some other element, so it must be separated first.
(e) While using H2 - O2 fuel cell in an
automobile, a high pressure must be created inside the engine,
which is risky.
15. What is lithium-ion battery?
Lithium-ion battery is a secondary battery. As in lithium cell, it
does not contain metallic lithium as anode. As the name suggests, the movement
of lithium ions are responsible for charging and discharging.
16. What are the components of LIB?
• A positive electrode (Layers of lithium-metal oxide) (cathode)
• A negative electrode (Layers of porous carbon) (anode)
• An electrolyte (Polymer gel) (separator)
17. What are the advantages of lithium cell? (AU. Jan. 2018)
1. Lithium-ion batteries are high voltage and light weight batteries.
2. It is smaller in size.
3. It produces three time the voltage of Ni-Cd batteries.
4. It has none of the memory effect seen in Ni-Cd batteries.
18. What are the uses of lithium-ion cell?
It is used in cell phone, note PC, portable LCD TV, semiconductor
driven audio, etc.,
19. What are super capacitors?
Super capacitor is a high capacity capacitor with capacitance
value much higher than other capacitor. They store 10 to 100 times more energy
per unit volume and deliver charge much faster than batteries.
20. Mention some important applications of super capacitors.
Super capacitors are used in many power management applications
like,
1. Voltage stabilization in start/stop system
2. Energy harvesting
3. Kitchen appliances
4. Regenerative braking system
Engineering Chemistry: Unit V: b. Energy Storage Devices : Tag: Engineering Chemistry : Energy Storage Devices | Engineering Chemistry - Anna University 2 Marks Questions and Answers
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
