Engineering Chemistry: Unit I: Water and its Treatment
Anna University Long Questions and Answers
Water and its Treatment | Engineering Chemistry
Engineering Chemistry : UNIT I : Water and its Treatment : Part B - Anna University long Questions & Answers
Unit - I
Chapter 1
Water and its Treatment
Anna University Long Questions & Answers
Part - B
1. Define and explain the significance of the followings
(i) turbidity
(ii) hardness
(iii) pH
(i) Turbidity
Turbidity is the reduction of clarity of natural water due to the
presence of finely divided, insoluble impurities suspended in water.
Significance
(i) Turbidity affects the taste and odour of drinking water.
(ii) As turbidity affects the disinfection process, it must be removed.
(iii) Turbidity have many negative effects on aquatic life, it block
light to aquatic plants, aquatic organisms.
(iv) Turbidity affects the growth rate of algae.
(v) It increases water temperature because suspended particles
absorbs more heat.
(ii) Hardness
Hardness is the property (or) characteristics of water, which does
not produce lather with soap.
Types of hardness
Depending upon the types of dissolved salts present in water,
hardness of water can be classified into two types
1. Temporary hardness.
2. Permanent hardness.
1. Temporary hardness (or) Carbonate hardness (CH) (or) Alkaline
hardness
This is due to the presence of bicarbonates of calcium and
magnesium. It can be removed by (i) boiling the water (ii) adding lime to the
water.
2. Permanent hardness (or) Non-carbonate hardness (NCH) (or)
Non-alkaline hardness
This is due to the presence of chlorides and sulphates of calcium
and magnesium. It cannot be removed by boiling the water. But, it can be removed
by (i) Lime-soda process (ii) Zeolite process.
Significance of Hardness
1. Hardness affects the amount of soap that is needed to produce
foam (or) lather.
2. Hardness is very important in industrial uses, because it forms
scale in heat exchange equipment boilers and pipe lines.
3. Hardness mitigates metal toxicity because Ca2+ and
Mg2+ help keep fish from absorbing metals such as lead, arsenic and
cadmium into their blood stream.
(iii) pH
The hydrogen ion concentration is represented by the pH value,
which is defined as
pH = - log10[H+]
Significance of pH
(i) pH determines the solubility (amount that can be dissolved in
water).
(ii) It also determines the biological availability (amount that can
be utilized by aquatic life).
(iii) A rise (or) fall in pH can indicate chemical pollution (or)
acid rain. Many animals cannot live in water at a pH level below 5 (or) above
9.
2. What is meant by disinfection? How is it carried out? Explain
in details about the break point chlorination.
The process of destroying the harmful bacterias is known as
sterilisation or disinfection. The chemicals used for this purpose are called
disinfectants. This process can be carried out by the following methods.
1. By using ozone
Ozone is a powerful disinfectant and is readily absorbed by water.
Ozone is highly unstable and breaks down to give nascent oxygen.
O3 → O2 + [O]
The nascent oxygen is a powerful oxidising agent and kills the
bacterias.
Disadvantages
(a) This process is costly and cannot be used in large scale.
(b) Ozone is unstable and cannot be stored for long time.
2. By using ultraviolet (UV) radiations
UV rays are produced by passing electric current through mercury
vapour lamp. This is particularly useful for sterilizing water in swimming
pool.
Disadvantages
(i) It is costly.
(ii) Turbid water cannot be treated.
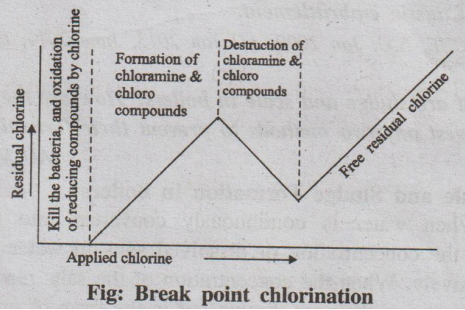
Fig: Break point chlorination
Chlorine may be added to water directly as a gas or in the form of
bleaching powder. When chlorine is applied to water, the results obtained can
be depicted graphically in the following Fig. The graph shows the relationship
between the amount of chlorine added to water and the residual chlorine.
It is observed from the graph that initially the applied chlorine
is used to kill the bacterias and oxidises all the reducing substances present
in the water and there is no free residual chlorine.
As the amount of applied chlorine increases, the amount of
combined residual chlorine also increases. This is due to the formation of
chloramine and other chloro compounds.
At one point, on further chlorination, the oxidation of
chloramines and other impurities starts and there is a fall in the combined
chlorine content.
Break point chlorination is the point at which the combined
residual chlorine decreases to a minimum point where oxidation of chloramines
and other impurities complete and free residual chlorine begins to appear.
Thus, the break point chlorination eliminates bacterias, reducing
substances, organic substances responsible for the bad taste and odour, from
the water.
3. Explain the following boiler troubles suggesting the remedical
methods: (i) Sludge and scale formation
(ii) Caustic embrittlement. (CBE. A.U. Jan 2009, AU Jan 2013, June 2014, Dec
2015)
(or)
What are sludge and scale in boilers? How are they formed Suggest
any two methods to prevent their formation. (AU May 2015)
(i) Scale and Sludge Formation in boilers
When water is continuously converted into steam in boilers, the
concentration of dissolved salts in water increases progressively. When the
concentration of the salts reaches their saturation point, they are thrown out
in the form of precipitates on the inner walls of the boilers. The least
soluble one gets precipitated first.
(a) Sludge
• If the precipitate is loose and slimy it is called sludge.
Sludges are formed by substances like MgCl2, MgCO3,
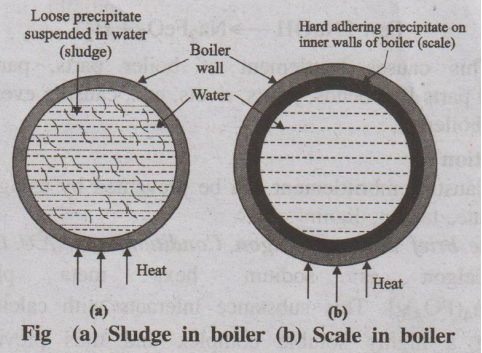
Fig (a) Sludge in boiler (b) Scale in boiler
MgSO4 and CaCl2. They have greater
solubilities in hot water than cold water.
(b) Scale
On the other hand, if the precipitate forms hard and adherent
coating on the inner walls of the boiler, it is called scale. Scales are formed
by substances like Ca(HCO3)2, CaSO4 and Mg(OH)2
Prevention of scale formation
1. Scales can be removed using scraper, wire brush etc.
2. They can be removed by thermal shocks.
3. By using suitable chemicals like dil. acids.
4. They can be removed by frequent blow down operation...
(ii) Caustic Embrittlement
Caustic embrittlement means intercrystalline cracking of boiler
metal.
Boiler water usually contains a small proportion of Na2CO3.
In high pressure boilers this Na2CO3 undergoes
decomposition to givé NaOH.
Na2CO3 + H2O → 2NaOH + CO2
This NaOH flows into the minute hair cracks and crevices, usually
present on the boiler material, by capillary action and dissolves the
surrounding area of iron as sodium ferroate.
Fe + 2NaOH → Na2FeO2 + H2↑
This causes brittlement of boiler parts, particularly stressed
parts like bends, joints, rivets, etc., causing even failure of the boiler.
Prevention
Caustic embrittlement can be prevented by using sodium phosphate,
tannin, lignin.
4. Write brief note on Calgon Conditioning. (A.U. Dec 2014)
Calgon is sodium hexa meta phosphate Na2 [Na4(PO3)6].
This substance interacts with calcium ions forming a highly soluble complex and
thus prevents the precipitation of scale forming salt.
2CaSO4 + Na2[Na4(PO3)6]
→ Na2 [Ca2(PO3)6] + 2a2SO4.
The complex Na2 [Ca2(PO3)6]
is soluble in water and there is no problem of sludge disposal. So calgon
conditioning is better than phosphate conditioning.
5. How will you protect boiler from corrosion. (A.U.Dec. 06)
(or)
What are the factors which causes boiler corrosion? How can it be
minimised. (A.U. Dec 2014)
(a) Chemical method
Sodium sulphite, hydrazine are some of the chemicals used for
removing oxygen.
2Na2SO3 + O2 → 2Na2SO4
N2H4 + O2 → N2 + 2H2O
(b) Mechanical de-aeration
Dissolved oxygen can also be removed from water by mechanical
deaeration (Fig.).
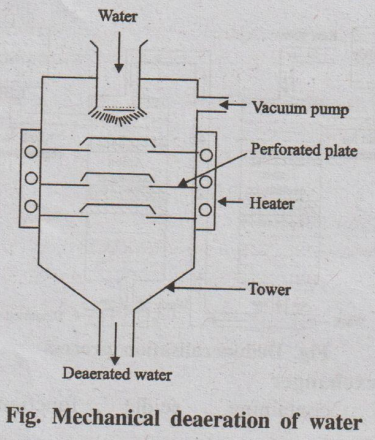 Fig. Mechanical deaeration of water
Fig. Mechanical deaeration of water
In this process, water is allowed to fall slowly on the perforated
plates fitted inside the tower. The sides of the tower are heated, and a vacuum
pump is also attached to it. The high temperature and low pressure produced
inside the tower reduce the dissolved oxygen content of the water.
(c) Removal of dissolved Carbon dioxide
(a) Carbon dioxide can be removed from water by adding a
calculated amount of NH4OH into water.
2NH4OH + CO2 → (NH4)2CO3 +
H2O
(b) Carbon dioxide along with oxygen can also be removed
mechanically by de-aeration method.
(d) Removal of acids
Corrosion by acids can be avoided by the addition of alkali to the
boiler water.
HCl + NaOH → NaCl + H2O
6. Explain the demineralization of water by ion-exchange process.
How are exhausted cation and anion exchange resins regenerated? (AU June 2017) (TNV A.U. Jan 2010, June 2016)
(or)
Draw a suitable diagram and describe the ion exchange process for
the softening of hard water. (A.U Dec
2015)
This process removes almost all the ions (both anions and cations)
present in hard water. Demineralisation process is carried out by using ion
exchange resins.
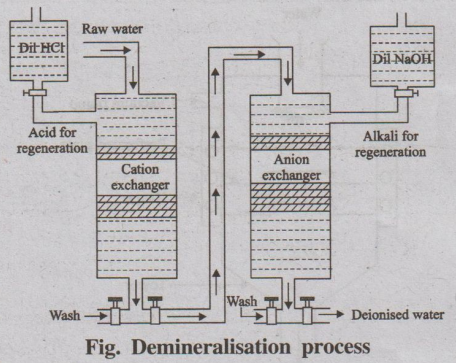
Fig. Demineralisation process
(i) Cation exchanger
Resins containing acidic functional groups (-COOH - SO3H)
are capable of exchanging their Ht ions with other cations of hard water. It is
represented as RH2.
(ii) Anion Exchanger
Resins containing basic functional groups (-NH2, -OH)
are capable of exchanging their anions with other anions of hard water. It is
represented as R (OH)2
Process
The hard water first passed through a cation exchange column, (Fig
which absorbs all the cations like Ca2+, Mg2+, Na+,
K+, etc., present in the hard water.
RH2 + CaCl2 → RCa + 2HC1
RH + NaCl → RNa + HCl
The cation free water is then passed through a anion exchange
column, which absorbs all the anions like Cl-, SO2-4,
HCO-3, etc., present in the water.
R'(OH)2 + 2HCl → R' Cl2 + 2H2O
The water coming out of the anion exchanger is completely free
from cations and anions. This water is known as demineralised water or
deionised water.
Regeneration
When the cation exchange resin is exhausted, it can be regenerated
by passing a solution of dil HCl or dil H2SO4.
RCa + 2HCl → RH2 + CaCl2
Similarly, when the anion exchange resin is exhausted, it can be
regenerated by passing a solution of dil NaOH.
R'Cl2 + 2NaOH → R'(OH)2 + 2NaCl.
(or)
7. How is the softening of water carried out using the zeolite
process? (AU June 2014, TCY A.U. Jan
2010, July 2016)
(or)
What are zeolites? How are they used in softening of water? Use a
diagram for your explanation. (A.U May
2015, Dec 2014)
(a) Zeolite
Zeolites are naturally occurring hydrated sodium aluminosilicate.
Its general formula is Na2O. Al2O3 . xSiO2
.yH2O.(x = 2 - 10, y = 2 - 6) Natural zeolites are green sand and
non-porous. The synthetic form of zeolite is known as permutit, which is porous
and possess gel like structure, hence it is generally used for water softening.
(b) Zeolite process
Zeolites is porous and possess gel like structure, hence it is
generally used for water softening. It is represented by Na2Ze. The
sodium ions which are loosely held in Na2Ze are replaced by Ca2+
and Mg2+ ions present in the water.
Process
When hard water is passed through a bed of sodium zeolite (Na2Ze),
kept in a cylinder (Fig.), it exchanges its sodium ions with Ca2+ and
Mg2+ ions present in the hard water to form calcium
and magnesium zeolites. The various reactions taking place during softening
process are
CaSO4 + Na2Ze → CaZe + Na2SO4
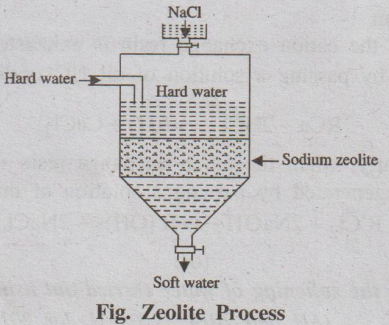
Fig. Zeolite Process
The softened water is enriched with large amount of sodium salts,
which do not cause any hardness, but cannot be used in boilers.
Regeneration
After some time zeolite gets exhausted. The exhausted zeolite is
again regenerated by treating with 10% solution of NaCl.
CaZe + 2NaCl → Na2Ze + CaCl2
Advantages of Zeolite process
(i) Water will have only hardness of 1-2 ppm.
(ii) No sludge is formed during this process.
(iii) Its operation is easy.
Disadvantages of Zeolite process
(i) Turbid water cannot be treated.
(ii) Acidic water cannot be treated.
(iii) Water containing Fe, Mn cannot be treated.
8. Explain the internal conditioning of water? Take two examples
for your explanation. (A.U May 2015)
(or) What is meant by internal conditioning of water (A.U. June 2017)
It is the process of removal of scale forming substance, which
were not completely removed in the external treatment, by adding chemicals
directly into the boiler.
(i) Colloidal conditioning
Scale formation can be avoided by adding colloidal conditioning
agents like kerosene, agar-agar, gelatin, etc., It is used in low pressure
boilers. These colloidal substances get coated over the scale forming particles
and convert them into non-adherent, loose precipitate called sludge, which can
be removed by blow down operation.
(ii) Phosphate conditioning
Scale formation can be avoided by adding sodium phosphate. It is
used in high pressure boilers. The phosphate reacts with Ca2+ and Mg2+
salts to give soft sludges of calcium and magnesium phosphates.-.
3 CaSO4 + 2 Na3PO4 → Ca3(PO4)2
+ 3Na2SO4
Generally 3 types of phosphates are employed.
(a) Trisodium phosphate - Na3PO4
(Too alkaline) - used for too acidic water.
(b) Disodium hydrogen phosphate - Na2HPO4 (weakly alkaline) - used for
weakly acidic water.
(c) Sodium dihydrogen phosphate - NaH2PO4 (acidic) - used for alkaline
water.
9. What is desalination? Explain any one method of desalination (Coim A.U. Jan 2010, A.U. Jan 2013)
(or)
What is reverse osmosis? How wil you purify the sea water by
reverse osmosis? Mention its advantages.
(TNV A.U. Jan 2010, Chen A.U. Dec 2014, May 2015)
(or)
Explain with neat diagram, the desalination of brackish water of
reverse osmosis method. (A.U. May
2017)
Desalination
The process of removing common salt (sodium chloride) from the
water is known as desalination.
Reverse Osmosis
When two solutions of different concentrations are separated by a
semi-permeable membrane, solvent (water) flows from a region of lower
concentration to higher concentration.
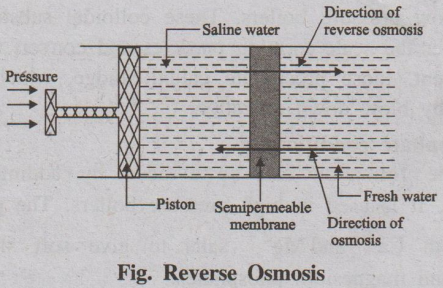
Fig. Reverse Osmosis
This process is called osmosis. The driving force in this
phenomenon is called osmotic pressure.
If a hydrostatic pressure in excess of osmotic pressure is applied
on the higher concentration side, the solvent flow is reversed i.e., solvent
flows from higher concentration to lower concentration. This process is called
reverse osmosis (Fig.). Thus, in the process of reverse osmosis pure water is
separated from salt water. This process is also known as super-filtration.
The membranes used are
Examples:
cellulose acetate, cellulose butyrate, etc.
Advantages
(i) The life time of the membrane is high, and it can be replaced
within few minutes.
(ii) It removes ionic as well as non-ionic, colloidal impurities.
(iii) Due to low capital cost, simplicity, low operating, this
process is used for converting sea water into drinking water.
Engineering Chemistry: Unit I: Water and its Treatment : Tag: Engineering Chemistry : Water and its Treatment | Engineering Chemistry - Anna University Long Questions and Answers
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
