Engineering Chemistry: Unit II: Nanochemistry
Anna University Long Questions and Answers
Nanochemistry | Engineering Chemistry
Engineering Chemistry : UNIT II : Nanochemistry : Anna University long Questions & Answers
Unit - II
Chapter 2
Nanochemistry
Anna University Long Questions & Answers
Part - B
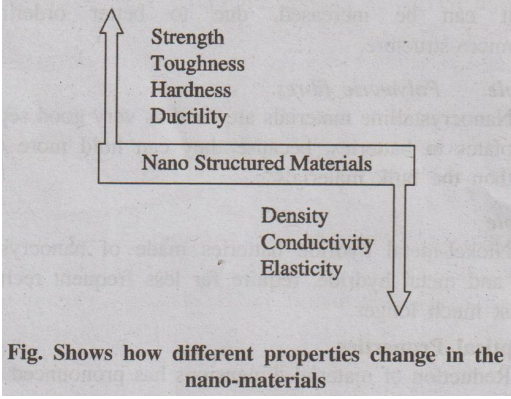
1. Explain about size dependent properties of nano-materials. (A.U. (CEG) Dec 2012, June 2014)
Nearly all the properties as shown in the following figure, like
hardness, strength, ductility, melting point and density, change for
nanomaterials. These behaviors vary so significantly by a mere reduction in
grain size. Nanomaterials are composed of grains and grain boundaries.
Nanometre sized grains contains only a few thousands of atoms with in each grain.
A large number of atoms reside at the grain boundaries. As the
grain size decreases, there is a significant increase in the volume fraction of
grain boundaries or interfaces.
The properties of the materials are bound to be governed to a
large extent by defect configurations. Hence the mechanical and chemical
properties of nanomaterials are significantly altered due to defect dynamics.
The elastic property of nanomaterials are different from that of bulk alloys
due to the presence of increased fraction of defects.
Example
1. Nanocrystalline ceramics are tougher and stronger than those
with coarse grains.
2. Write an informative note on the properties of nano-particles. (A.U. (CEG) June 2013)
(Or)
Discuss any four salient properties of nano-materials. (A.U. June 2014, Dec 2015)
1. Electrical Properties
(i) Electrical conductivity decreases with a reduced dimension
due to increased surface scattering. However, it can be increased, due to
better ordering in micro-structure.
Example : Polymeric fibres.
(ii) Nanocrystalline materials are used as very good separator
plates in batteries, because they can hold more energy than the bulk
materials.
Example
Nickel-metal hydride batteries made of nanocrystalline nickel and
metal hydride, require far less frequent recharging and last much longer.
2. Optical Properties
Reduction of material dimensions has pronounced effects on the
optical properties. Optical properties of nano-materials are different from
bulk forms.
The change in optical properties is caused by two factors
(i) The quantum confinement of electrons within the nano-particles
increases the energy level spacing.
Example
The optical absorption peak of a semiconductor nano-particles
shifts to a short wavelength, due to an increased band gap.
(ii) Surface plasma resonance, which is due to smaller size of
nano-particles than the wavelength of incident radiation.
Example
The colour of metallic nano-particles may change with their sizes
due to surface plasma resonance.
3. Mechanical properties
The nano-materials have less defects compared to bulk materials,
which increases the mechanical strength.
(i) Mechanical properties of polymeric materials can be increased
by the addition of nano-fillers.
(ii) As nano-materials are stronger, harder and more wear
resistant and corrosion resistant, they are used in spark plugs.
Example:
Nano-crystalline carbides are much stronger, harder and wear
resistant and are used in micro drills.
4. Magnetic properties
Magnetic properties of nano materials are different from that of
bulk materials. Ferro-magnetic behaviour of bulk materials disappear, when the
particle size is reduced and transfers to super-paramagnetics. This is due to
the huge surface area.
3. Discuss the laser ablation and CVD techniques for the synthesis
of nanoparticles. (A.U (CEG) June 2013,
A.U. Jan 2015)
(i) Laser ablation
In laser ablation technique, high-power laser pulse is used to
evaporate the material from the target. The stoichiometry of the material is
protected in the interaction.
The total mass ablated from the target per laser pulse is referred
as the ablation rate.
This method involves vapourisation of target material containing
small amount of catalyst (nickel or cobalt) by passing an intense pulsed laser
beam at a higher temperature to about 120°C in a quartz tube reactor.
Simultaneously, an inert gas such as argon, helium is allowed to pass into the
reactor to sweep the evaporated particles from the furnace to the colder
collector.
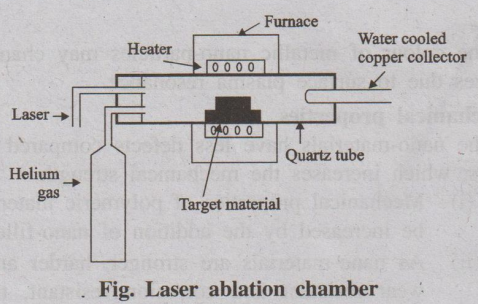
Fig. Laser ablation chamber
(ii) Chemical Vapour Deposition (CVD)
This process involves conversion of gaseous molecules into solid
nanomaterials in the form of tubes, wires or thin films. First the solid
materials are converted into gaseous molecules and then deposited as
nanomaterials.
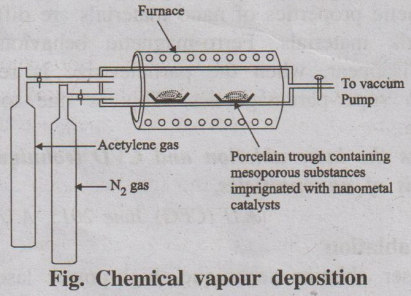 Fig. Chemical vapour deposition
Fig. Chemical vapour deposition
Example: CNT preparation.
The CVD reactor consists of a higher temperature vacuum furnace
maintained at inert atmosphere. The solid substrate containing catalyst like
nickel, cobalt, iron supported on a substrate material like, silica, quarts is
kept inside the furnace. The hydrocarbons such as ethylene, acetylene and
nitrogen cylinders are connected to the furnace. Carbon atoms, produced by the
decomposition at 1000°C, condense on the cooler surface of the catalyst.
As this process is continuous, CNT is produced continuously.
4. What are nano-particles? Write any four methods of preparation
of nano-particles? (A.U. (CEG) Dec 2011)
(Or)
Discuss various types of synthesis involved in the preparation of
nano-materials. (A.U. Jan 2014, June 2014)
Definition
Nanoparticles are the particles, the size of which ranges from
1-50 nm.
1. Solvothermal Synthesis
Solvothermal synthesis involves the use of solvent under high
temperature (between 100°C to 1000°C) and moderate to high pressure (1 atm to
10,000 atm) that facilitate the interaction of precursors during synthesis.
Method
A solvent like ethanol, methanol, 2-propanol is mixed with certain
metal precursors and the solution mixture is placed in an autoclave kept at
relatively high temperature and pressure in an oven to carry out the crystal
growth. The pressure generated in the vessel, due to the solvent vapour,
elevates the boiling point of the solvent.
Example: Solvothermal synthesis of
zinc oxide
Solvothermal synthesis of zinc oxide
Zinc acetate dihydrate is dissolved in 2-propanol at 50°C.
Subsequently, the solution is cooled to 0°C and NaOH is added to precipitate
ZnO. The solution is then heated to 65°C to allow ZnO growth for some period of
time. Then a capping agent (1-dodecanethiol) is injected into the suspension to
arrest the growth. The rod shaped ZnO nano-crystal is obtained.
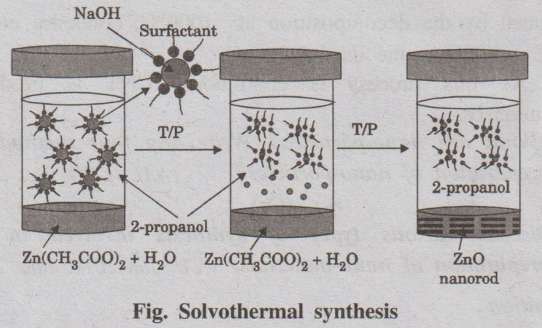
Fig. Solvothermal synthesis
2. Laser ablation
In laser ablation technique, high-power laser pulse is used to
evaporate the material from the target. The stoichiometry of the material is
protected in the interaction. .. The total mass ablated from the target per
laser pulse is referred to as the ablation rate.
This method involves vapourisation of target material containing
small amount of catalyst (nickel or cobalt) by passing an intense pulsed laser
beam at a higher temperature to about 120°C in a quartz tube reactor.
Simultaneously, an inert gas such as argon, helium is allowed to pass into the
reactor to sweep the evaporated particles from the furnace to the colder
collector.
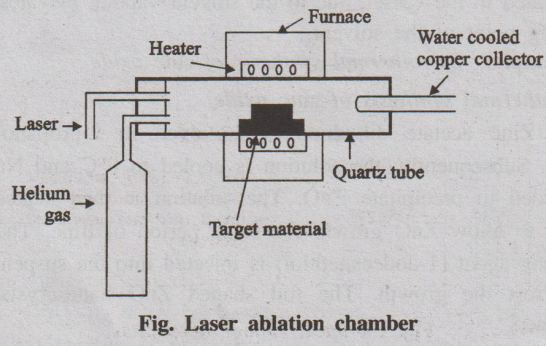
Fig. Laser ablation chamber
Uses
1. Nanotubes having a diameter of 10 to 20 nm and 100 um can be
produced by this method.
2. Ceramic particles and coating can be produced.
3. Other materials like silicon, carbon can also be converted into
nanoparticles by this method.
3. Chemical Vapour Deposition (CVD)
This process involves conversion of gaseous molecules into solid
nanomaterials in the form of tubes, wires or thin films. First the solid
materials are converted into gaseous molecules and then deposited as
nanomaterials.
Example: CNT preparation.
The CVD reactor consists of a higher temperature vacuum furnace
maintained at inert atmosphere. The solid substrate containing catalyst like
nickel, cobalt, iron supported on a substrate material like, silica, quarts is
kept inside the furnace. The hydrocarbons such as ethylene, acetylene and
nitrogen cylinders are connected to the furnace. Carbon atoms, produced by the
decomposition at 1000°C, condense on the cooler surface of the catalyst.
As this process is continuous, CNT is produced continuously.
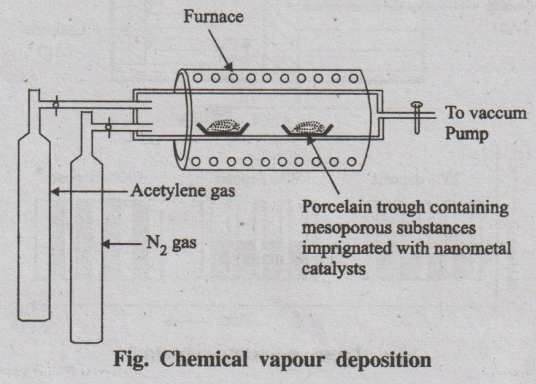
Fig. Chemical vapour deposition
4. Electro-deposition (or) Electrochemical deposition
Electro-deposition is an electrochemical method in which ions from
the solution are deposited at the surface of cathode.
Process of electro-deposition
The cell consists of a reference electrode, specially designed
cathode and anode. All these electrodes are connected with the battery through
an voltmeter and dipped in an electrolytic solution of a soluble metal as shown
in figure. When the current is passed through the electrodes of template, the
metal ions from the solution enter into the pores and gets reduced at the
cathode, resulting in the growth of nanowire inside the pores of the template.
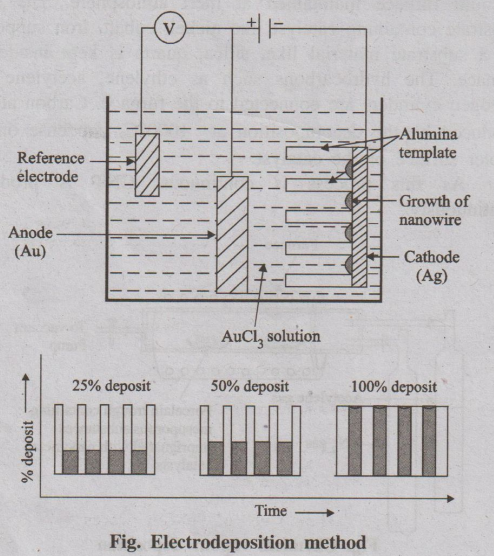
Fig. Electrodeposition method
5. Explain the preparation of nano-materials by (i) Sol-Gel
process (ii) Electrospinning
1. Sol-Gel process
The sol-gel process is a wet chemical technique also known as
chemical solution deposition. It is the method for producing solid materials
from small molecules. This method is used for the fabrication of metal oxides.
It involves conversion of monomers into a colloidal solution (sol), that acts
as the precursor. This colloidal solution gradually evolves towards the
formation of a gel-like system.
It involves the following steps.
1. Hydrolysis and polycondensation 2. Gelation 3. Aging 4. Drying
5. Densification 6. Crystallization
The volume fraction of particles (particle density) may be slow
that a significant amount of fluid need to be removed for the gel-like
properties to be recognized. It is done by two ways.
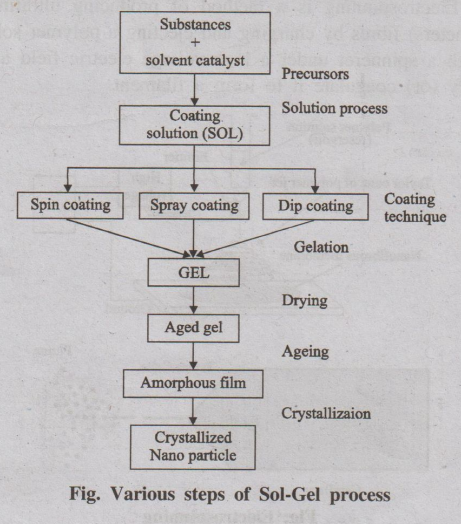
Fig. Various steps of Sol-Gel process
(i) Sedimentation
The solution is allowed to keep for some time for sedimentation to
occur and then pour off the remaining liquid.
(ii) Centrifugation
Centrifugation can also be used to accelerate the process of phase
separation.
Drying and densification
Removal of the remaining liquid (solvent) is done by drying
process, which accompanied by shrinkage and densification.
Firing (or) crystallization
A thermal treatment (firing) is necessary to enhance mechanical
properties and structural stability via sintering, densification
2. Electrospinning
Electrospinning is a method of producing ultrafine (in nanometers)
fibres by charging and ejecting a polymer solution through a spinneret under a
high-voltage electric field and to solidify (or) coagulate it to form a
filament.
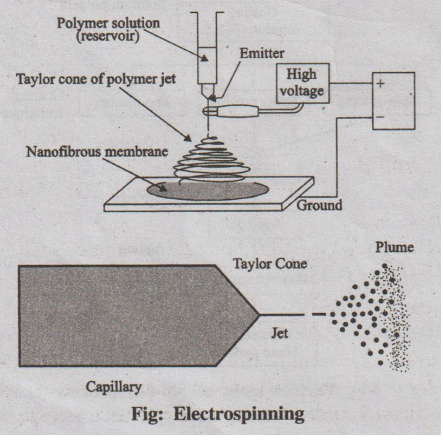
Components
1. A high voltage power supply.
2. A polymer reservoir that can maintain a constant flow rate of
solution.
3. A conductive needle, as polymer source, connected to the high
voltage power supply.
4. A conductive collector (plate, drum, etc.)
Process
A polymer is dissolved in a suitable solvent and is filled in the
capillary reservoir. When sufficiently high voltage is applied to create an
electric field between the needle tip and the collector, a charge accumulates
at the liquid surface. When the electrostatic repulsion is higher than the
surface tension the liquid meniscus is deformed into conically shaped structure
known as a Taylor cone.
Once the Taylor cone is formed, the charged liquid jet is ejected
towards the collector. Depending upon the viscosity of the solution, solid
fibre will be formed as the solvent evaporates.
6. Define the terms: nanorods, nanotubes, nanowires and
nanoclusters. (A.U (CEG) June 2013)
(a) Nano-wires
Nanowire is two dimensional cylindrical solid material having an
aspect ratio ie., length to width ratio greater than 20. Diameter of the
nanowire ranges from 10 - 100 nm.
(b) Nano-rods
Nanorod is two dimensional cylindrical solid material having an
aspect ratio i.e., length to width ratio less than
(c) Nano Cluster
Nanoclusters are fine aggregates of atoms or molecules. The size
of which ranges from 0.1 to 10 nm. Of all the nano materials, nanoclusters are
the smallest sized nano materials because of their close packing arrangement of
atoms.
(d) Nano-tubes
Nanotubes are tube like structures with diameter of 1 - 100 nm and
a length of few nm to microns. Nanotubes consist of tiny cylinders of carbon
and other materials like boron nitride. Nanotubes may be organic (or)
inorganic.
7. What are nano materials? Discuss the types of carbon nano tubes
and their applications. (A.U June
2012)
Nanomaterials are the materials having components with size less
than 100 nm at least in one dimension.
Types of carbon nanotubes
(i) Single - walled nanotubes (SWNTS)
SWNTs consist of one tube of graphite. It is one-atom thick having
a diameter of 2 nm and a length of 100 um. SWNTs are very important, because
they exhibit important electrical properties. It is an excellent conductor.
Three kinds of nanotubes are resulted, based on the orientation of
the hexagon lattice.
(a) Arm-chair structures: The
lines of hexagons are parallel to the axis of the nanotube.
(b) Zig-zag structures: The
lines of carbon bonds are down the centre.
(c) Chiral nanotubes: It
exhibits twist or spiral around the nanotubes.
It has been confirmed that arm-chair carbon nanotubes are metallic
while zig-zag and chiral nanotubes are semiconducting
(ii) Multi - walled nanotubes (MWNTs)
MWNTs (nested nanotubes) consist of multiple layers of graphite
rolled in on themselves to form a tube shape. It exhibits both metallic and
semiconducting properties. It is used for storing fuels such as hydrogen and
methane.
Applications of Carbon Nanotubes
(a) It is used in battery technology and in industries as
catalyst.
(b) It is also used as light weight shielding materials for
protecting electronic equipments.
(c) CNTs are used effectively inside the body for drug
delivery.
(d) It is used in composites, ICs..
8. How are carbon nano tubes synthesized? What are its
applications? (A.U (CEG) Dec 2011, AU. Jan
2014, Jan 2015)
Carbon nanotubes can be synthesized by any one of the following
methods.
(i) Pyrolysis of hydrocarbons.
(ii) Laser evaporation.
(i) Pyrolysis
Carbon nanotubes are synthesized by the pyrolysis of hydrocarbons
such as acetylene at about 700°C in the presence of Fe-silica or Fe-graphite
catalyst under inert conditions.
(ii) Laser evaporation
It involves vapourization of graphite target, containing small
amount of cobalt and nickel, by exposing it to an intense pulsed laser beam at
higher temperature (1200°C) in a quartz tube reactor. An inert gas such as
argon is simultaneously allowed to pass into the reactor to sweep the
evaporated carbon atoms from the furnace to the colder copper collector, on
which they condense as carbon nanotubes.
Applications of CNTs
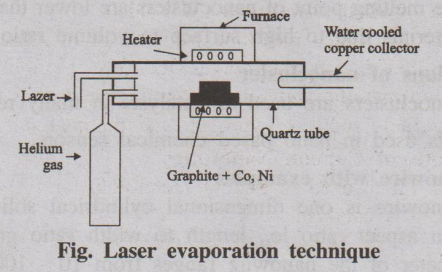
Fig. Laser evaporation technique
9. Explain (a) Nanocluster (b) Nanowire with examples (A.U Jan 2014)
(a) Nanocluster
Nanoclusters are fine aggregates of atoms or molecules. The size
of which ranges from 0.1 to 10 nm. Of all the nano materials, nanoclusters are
the smallest sized nano materials because of their close packing arrangement of
atoms.
Examples: CdS, ZnO, etc.,
All the atoms, in nanocluster, are bound by forces like metallic,
covalent, ionic, hydrogen bond' or Vander Waals forces of attraction. Clusters
of certain critical size are more stable than others. Nanoclusters consisting
of up to a couple of hundred atoms, but larger aggregates, containing 10° or
more atoms, are called nanoparticles.
Magic number
Magic number is the number of atoms present in the clusters of
criticle sizes with higher stability.
Production of Nanoclusters
Nanoclusters can be produced from atomic (or) molecular
constituents (or) from the bulk materials either by bottom up process (or) top
down process.
Atomic clusters (or) molecular clusters are formed by the
nucleation of atoms (or) molecules respectively.
Properties of nanoclusters
(i) The reactivity of nanoclusters are decreased due to their decrease
in size.
(ii) The melting point of nanoclusters are lower than the bulk materials
due to high surface to volume ratio.
Applications of nanocluster
(i) Nanoclusters are used as catalysts in many reactions.
(ii) It is used in nano based chemical sensors.
(b) Nanowire with examples
Nanowire is one dimensional cylindrical solid material having an
aspect ratio ie., length to width ratio greater than 20. Diameter of the
nanowire ranges from 10 - 100 nm.
Synthesis of nanowires
Template-assisted synthesis
Template assisted synthesis of nanowires is a simple way to
fabricate nanostructures. These templates contain very small cylindrical pores
or voids within the host material and the empty spaces are filled with the
chosen material to form nanowires.
Properties of nanowires
(i) Nanowires are two-dimensional material.
(ii) Conductivity of a nanowire is less than that of the
corresponding bulk materials.
Uses of nanowires
(i) Nanowires are used for enhancing mechanical properties of
composites.
(ii) It is also used to prepare active electronic components such
as p-n junction and logic gates.
10. Discuss the solvo thermal and laser ablation methods of
synthesis of nano-materials. (A.U. May
2015) Refer Q.No. 4 Page No B5.
Definition
Nanoparticles are the particles, the size of which ranges from
1-50 nm.
1. Solvothermal Synthesis
Solvothermal synthesis involves the use of solvent under high
temperature (between 100°C to 1000°C) and moderate to high pressure (1 atm to
10,000 atm) that facilitate the interaction of precursors during synthesis.
Method
A solvent like ethanol, methanol, 2-propanol is mixed with certain
metal precursors and the solution mixture is placed in an autoclave kept at
relatively high temperature and pressure in an oven to carry out the crystal
growth. The pressure generated in the vessel, due to the solvent vapour,
elevates the boiling point of the solvent.
Example: Solvothermal synthesis of
zinc oxide
Solvothermal synthesis of zinc oxide
Zinc acetate dihydrate is dissolved in 2-propanol at 50°C.
Subsequently, the solution is cooled to 0°C and NaOH is added to precipitate
ZnO. The solution is then heated to 65°C to allow ZnO growth for some period of
time. Then a capping agent (1-dodecanethiol) is injected into the suspension to
arrest the growth. The rod shaped ZnO nano-crystal is obtained.

Fig. Solvothermal synthesis
2. Laser ablation
In laser ablation technique, high-power laser pulse is used to
evaporate the material from the target. The stoichiometry of the material is
protected in the interaction. .. The total mass ablated from the target per
laser pulse is referred to as the ablation rate.
This method involves vapourisation of target material containing
small amount of catalyst (nickel or cobalt) by passing an intense pulsed laser
beam at a higher temperature to about 120°C in a quartz tube reactor.
Simultaneously, an inert gas such as argon, helium is allowed to pass into the
reactor to sweep the evaporated particles from the furnace to the colder
collector.

Fig. Laser ablation chamber
Uses
1. Nanotubes having a diameter of 10 to 20 nm and 100 um can be
produced by this method.
2. Ceramic particles and coating can be produced.
3. Other materials like silicon, carbon can also be converted into
nanoparticles by this method.
3. Chemical Vapour Deposition (CVD)
This process involves conversion of gaseous molecules into solid
nanomaterials in the form of tubes, wires or thin films. First the solid
materials are converted into gaseous molecules and then deposited as
nanomaterials.
Example: CNT preparation.
The CVD reactor consists of a higher temperature vacuum furnace
maintained at inert atmosphere. The solid substrate containing catalyst like
nickel, cobalt, iron supported on a substrate material like, silica, quarts is
kept inside the furnace. The hydrocarbons such as ethylene, acetylene and
nitrogen cylinders are connected to the furnace. Carbon atoms, produced by the
decomposition at 1000°C, condense on the cooler surface of the catalyst.
As this process is continuous, CNT is produced continuously.

Fig. Chemical vapour deposition
4. Electro-deposition (or) Electrochemical deposition
Electro-deposition is an electrochemical method in which ions from
the solution are deposited at the surface of cathode.
Process of electro-deposition
The cell consists of a reference electrode, specially designed
cathode and anode. All these electrodes are connected with the battery through
an voltmeter and dipped in an electrolytic solution of a soluble metal as shown
in figure. When the current is passed through the electrodes of template, the
metal ions from the solution enter into the pores and gets reduced at the
cathode, resulting in the growth of nanowire inside the pores of the template.

Fig. Electrodeposition method
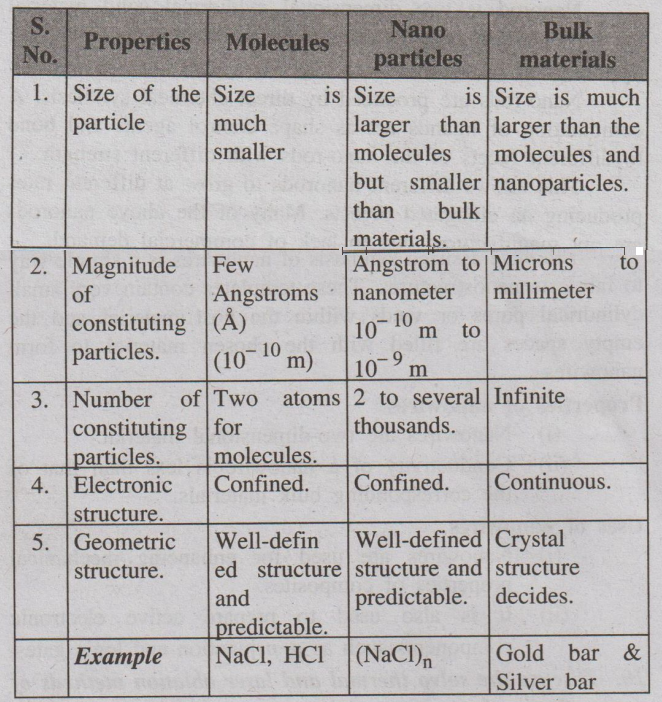
11. Compare the properties of molecules, nanoparticles and bulk
materials. (A.U May 2015)
(or)
Distinguish molecules, nanoparticles and bulk materials. (A.U Jan 2014)
12. (a) Write short notes on: (i) Carbon nanotubes (ii) Nanorods
(iii) Nanowires (A.U Dec 2015)
(i) Carbon nanotubes
Nanomaterials are the materials having components with size less
than 100 nm at least in one dimension.
Types of carbon nanotubes
(i) Single - walled nanotubes (SWNTS)
SWNTs consist of one tube of graphite. It is one-atom thick having
a diameter of 2 nm and a length of 100 um. SWNTs are very important, because
they exhibit important electrical properties. It is an excellent conductor.
Three kinds of nanotubes are resulted, based on the orientation of
the hexagon lattice.
(a) Arm-chair structures: The
lines of hexagons are parallel to the axis of the nanotube.
(b) Zig-zag structures: The
lines of carbon bonds are down the centre.
(c) Chiral nanotubes: It
exhibits twist or spiral around the nanotubes.
It has been confirmed that arm-chair carbon nanotubes are metallic
while zig-zag and chiral nanotubes are semiconducting
(ii) Multi - walled nanotubes (MWNTs)
MWNTs (nested nanotubes) consist of multiple layers of graphite
rolled in on themselves to form a tube shape. It exhibits both metallic and
semiconducting properties. It is used for storing fuels such as hydrogen and
methane.
Applications of Carbon Nanotubes
(a) It is used in battery technology and in industries as
catalyst.
(b) It is also used as light weight shielding materials for
protecting electronic equipments.
(c) CNTs are used effectively inside the body for drug
delivery.
(d) It is used in composites, ICs..
(ii). Nanorods
Nanorod is two dimensional cylindrical solid material having an
aspect ratio i.e., length to width ratio less than 20.
Synthesis of nanorods
Nano-rods are produced by direct chemical synthesis. A combination
of ligands act as shape control agents and bond to different facets of the
nano-rods with different strength.
This allows different nanorods to grow at different rates
producing an elongated objects. Many of the above nanorods are not manufactured
due to lack of commercial demand.
Properties of nanorods
1. Nanorods are two-dimensional materials.
2. It exhibits optical and electrical properties.
Applications of nanorods
1. Nanorods find application in display technologies.
2. It is also used in the manufacturing of micro mechanical switches.
3. They are used in energy harvesting and light emitting devices.
4. Nanorods have used as
cancer therapeutics.
(iii) Nanowires
Nanowire is two dimensional cylindrical solid material having an
aspect ratio ie., length to width ratio greater than 20. Diameter of the
nanowire ranges from 10 - 100 nm.
Synthesis of nanowires
1. Template-assisted synthesis
Template assisted synthesis of nanowires is a simple way to
fabricate nanostructures. These templates contain very small cylindrical pores
or voids within the host material and the empty spaces are filled with the
chosen material to form nanowires.
2. VLS (Vapour - Liquid - Solid) method
It involves the absorption of the source material from the gas
phase into a liquid phase of catalyst. Upon supersaturation of the liquid
alloy, a nucleation event generates a solid precipitate of the source material.
This seed serves as a preferred site for further deposition of material at the
interface of the liquid droplet, promoting the elongation of the seed into a
nanowire.
Properties of nanowires
1. Nanowires are two-dimensional material.
2. Conductivity of a nanowire is less than that of the
corresponding bulk materials.
3. Silicon nanowires show strong photoluminescence characteristics.
Uses of nanowires
1. Nanowires are used for enhancing mechanical properties of
composites.
2. It is also used to prepare active electronic components such as
p-n junction and logic gates.
13. Explain briefly the applications of nano-materials. (A.U.T (TNV) Jan 2009)
(Or)
Explain any sir applications of nano-materials in various fields. (A.U. May 2014)
I. Medicine
1. Nano drugs
Nano materials are used as nano drugs for the cancer and TB
therapy,
2. Laboratories on a chip
Nano technology is used in the production of laboratories on a
chip.
3. Nano-medibots
Nano particles function as nano-medibots that release anti-cancer
drug and treat cancer.
4. Gold-coated nanoshells
It converts light into heat, enabling the destruction of tumours.
II. In Agriculture
1. They also minimize the amount of harmful chemicals that pollute
the environment.
2. Nanosensors are used in crop protection for the identification
of diseases and residues of agrochemicals.
3. Nanodevises are used for the genetic engineering of plants.
III. In Energy
1. Power generation
Sun light, concentrated on nanoparticles, can produce steam with
high energy efficiency, which can even be used in running power plants.
2. Generating hydrogen from sea water
The use of a nanostructured thin film of nickel selenide as a
catalyst for the electrolysis of hydrogen from sea water.
3. Producing high efficiency light bulbs
Nano-engineered polymer matrix is used for the production of high
efficiency light bulbs.
IV. Electronics
1. Quantum wires are found to have high electrical conductivity.
2. The integrated memory circuits have been found to be effective
devices.
3. Nano wires are used to build transistors without p-n junctions.
V. In Catalysis
1. Water purification
Nanosilver catalyst is highly efficient in controlling microbes in
water.
2. Bio-diesel production
Solid base nanocatalyst KF/CaO can be used for biodiesel
production with yield more than 96%.
3. Fuel cell application
Carbon supported electro-catalysts play an important role in fuel
cell.
4. Gold nanoparticles
It is an important catalyst in co-oxidation, epoxidation of
propylene, hydrogenation of unsaturated hydrocarbons.
Engineering Chemistry: Unit II: Nanochemistry : Tag: Engineering Chemistry : Nanochemistry | Engineering Chemistry - Anna University Long Questions and Answers
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
