Engineering Chemistry: Unit V: a. Energy Sources
Anna University Long Questions and Answers
Energy Sources | Engineering Chemistry
Engineering Chemistry : UNIT V : Energy Sources and storage devices : Anna University long Questions & Answers
Unit - V
Chapter 7
Energy Sources
Anna University Long Questions & Answers
Part - B
1. Define mass defect and binding energy? How is mass defect calculated?
(i) Mass defect
The difference between the calculated and experimental masses of
nucleus is called mass defect. It is denoted by ∆m.
∆m = {Total mass of the protons, neutrons and electron } –
{Experimental mass of the nucleus }
(or)
It is defined as the loss of mass during the formation of the
nucleus of the isotope.
(ii) Binding energy
Binding energy is defined as the energy released when a given
number of protons and neutrons coalesee to form nucleus.
(or)
It is the energy required to disrupt the nucleus into its
constituent protons and neutrons.
Calculation of mass defect
Consider an isotope,
let its atomic number = Z
Mass number = A
If its atom contains
Z protons, Z electrons and (A-Z) neutrons
Let,
mp = mass of proton
mn = mass of neutron
me = mass of an electron
ஃ Calculated
mass of isotope
M' =Zmp + Zme + (A - Z)mn
= ZmH + (A - Z) mn
(where, mp + me = mass of H atom = mH)
Let,
M = Actual experimental mass of the nucleus,
then, the mass defect (∆m) =M' - M
(or)
∆m = ZmH + (A - Z) mn - M.
2. Calculate the mass defect of 2He4, if its
experimentally determined mass is 4.00390 amu. The masses of a proton, an
electron and a neutron are 1.007825, 0.0005852 and 1.008668 amu respectively.
Solution
Mass defect (∆m) of He-atom = [2mp + 2me +
2mn - M]
Note: 2He4 atom
is composed of 2 protons, 2 electrons and 2 neutrons.
Given: mp = 1.007825 amu; me = 0.0005852 amu
;
mn = 1.008668 amu
ஃ ∆m= [2 ×
1.007825 + 2 × 0.0005852 + 2 × 1.008688 – 4.00390] amu
= [4.0341964 – 4.00390] amu
= 0.0302964 amu
3. What are the components of a nuclear reactor? Write briefly
about each component. (Chen. A.U. June 2009)
(Or)
Explain with a neat diagram the parts and functions of a nuclear
reactor. (Chen. A.U. Jan 2010, May 2017)
(i) Fuel rods
The fissionable materials, used in the nuclear reactor as rods is
enriched U235.
Example: U235; Pu239
Function: It produces heat
energy and neutrons, that starts nuclear chain reaction.
(ii) Control rods
To control the fission reaction (rate), movable rods, made of
cadmium (or) boron, are suspended between fuel rods. These rods can be lowered
or raised and control the fission reaction by absorbing excess neutrons.
Examples : Cd113 ; B10
Function: It controls the
nuclear chain-reaction and avoids the damage of the reactors.
(iii) Moderators
The substances used to slow down the neutrons are called
moderators.
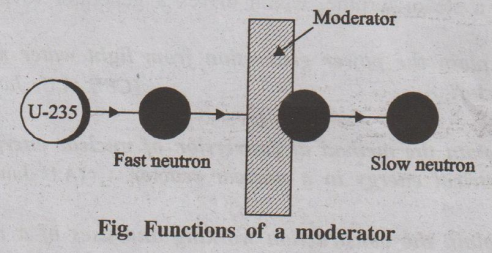
Fig. Functions of a moderator
When the fast-moving neutrons collide with moderator, they lose
energy and gets slow down.
Example:
Ordinary water, heavy water, graphite, beryllium. Function: The
kinetic energy of fast moving neutrons (1 meV) is reduced to slow neutrons
(0.25 eV).
(iv) Coolants
In order to absorb the heat produced during fission, a liquid
called coolant is circulated in the reactor core.
Example:
Water (act as moderator & coolant), heavy water.
Function: It cools the fuel
core.
(v) Pressure vessel
It encloses the core and also provides the entrance and exit
passages for coolant.
Function: It withstand the
pressure as high as 200 kg/cm2 .
(vi) Protective shield
The nuclear reactor is enclosed in a thick massive concrete shield
(more than 10 meters thick).
Function: The environment and
operating personnels are protected from destruction in case of leakage of
radiation.
(vii) Turbine
The steam generated in the heat exchanger is used to operate a
steam turbine, which drives a generator to produce electricity.
4. Explain the power generation from light water nuclear reactor. (CBE. A.U. Jan 2009)
(or)
Explain the method of conversion of nuclear energy into electrical
energy in a nuclear reactor. (A.U June
2014)
(or)
Explain the construction working and uses of a nuclear reactor
with a neat diagram. (A.U May 2015, Dec
2015, June 2016)
Light-water nuclear-power plant is the one, in which U235
fuel rods are submerged in water. Here the water acts as coolant and moderator.
Working
The fission reaction is controlled by inserting or removing the
control rods of B10 automatically from the spaces in between the
fuel rods. The heat emitted by fission of

Fig. Light water nuclear power plant
U235 in the fuel core is absorbed by the coolant (light
water). The heated coolant (water at 300°C) then goes to the heat exchanger
containing sea water. The coolant here, transfers heat to sea water, which is
converted into steam. The steam then drives the turbines, generating
electricity.
Pollution
Though nuclear power plants are very important for production of
electricity, they will cause a serious danger to environments.
Problem on disposal of reactor waste
The nuclear waste is packed in concrete barrels, which are buried
deep in the sea.
Uses of nuclear reactor
1. It is used to produce electricity.
2. It is used for propulsion (nuclear marine propulsion and rocket
propulsion)
3. It is used as a source of heat. It is also used as production
reactors for transmutation of elements (production of fissible materials, radio
active isotopes and materials for nuclear weapons).
5. It provides a source of neutron radiation and positron radiation.
5. Describe the breeder reactor. (Coim A.U. Jan 2010)
(or)
Write a detailed note on breeder reactors (A.U. June 2014, Dec 2014)
(or)
What is a breeder reactor? Describe with a neat diagram the
conversion of U-235 into Pu-239. (A.U July
2016)
Breeder reactor is the one which converts non-fissionable material
(U238, Th232) into fissionable material (U235,
Pu239). Thus the reactor produces or breeds more fissionable
material than it consumes.
Illustration
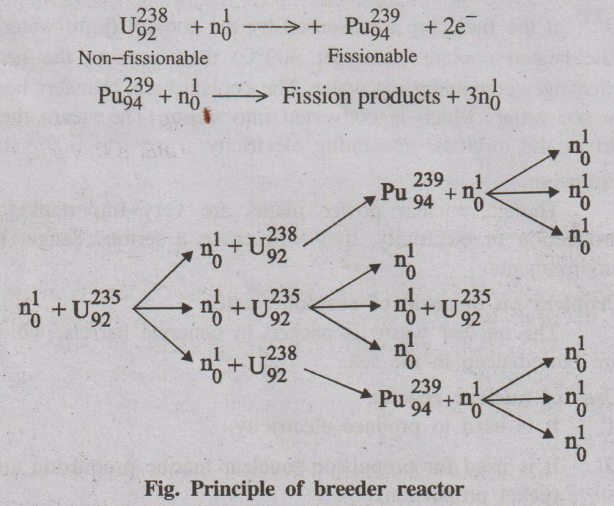
Fig. Principle of breeder reactor
In breeder reactor, of the three neutrons emitted in the fission
of U235, only one is used in propagating the fission chain with U235.
The other two are allowed to react with U238.
Thus, two fissionable atoms of Pu239 are produced for
each atom of U235 consumed. Therefore, the breeder reactor produces
more fissionable material than it uses. Hence Pu239 is a man made
nuclear fuel and is known as secondary nuclear fuel.
Significance
(i) The non-fissionable nucleides, such as U238 &
Th232, called fertile nucleides, are converted into fissile
nucleides.
(ii) The fissionable nucleides such as U235 & Pu239
are called fissile nucleides.
(iii) As regeneration of fissile nucleided takes place, its
efficiency is more.
6. What is a photovoltaic cell? Explain the construction and
working of a photovoltaic cell with a diagram. (A.U May 2015)
(or)
Describe the conversion of solar energy into electrical energy. (CBE. A.U. Jan 2009)
Definition
Photogalvanic cell (or) Photovoltaic cell is the one, which
converts the solar energy (energy obtained from the sun) directly into
electrical energy.
Construction
Solar cells consist of a p-type semiconductor (such as Si doped
with B) and n-type semiconductor (such as Si doped with P). They are in close
contact with each other.
Working
When the solar rays fall on the top layer of p-type semiconductor,
the electrons from the valence band get promoted to the conduction band and
cross the p-n junction into n-type semiconductor. There by potential difference
between two layers is created, which causes flow of electrons (ie., an electric
current). Thus, when this p and n layers are
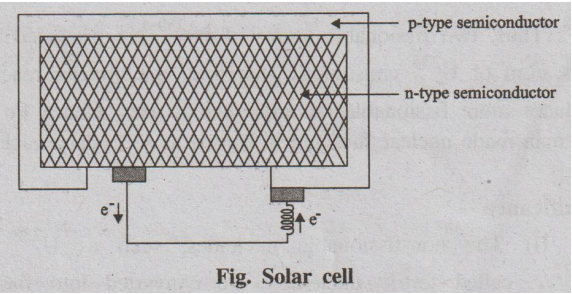
Fig. Solar cell
connected to an external circuit, electrons flow from n-layer to
p-layer, and hence current is generated.
The potential difference and hence current increases as more solar
rays falls on the surface of the top layer.
7. State the principle and applications of solar batteries (A.U. Jan 2013, May 2008, Dec 2014)
Principle
The basic principle involved in the solar cells is based on the
photovoltaic (PV) effect. When the solar rays fall on a two layer of
semi-conductor devices, a potential difference between the two layer is
produced. This potential difference causes flow of electrons and produces
electricity.
Applications of solar batteries
1. Lighting purpose
Solar battery can be used for lighting purpose. Now a days
electrical street lights are replaced by solar street lights.
2. Solar pumps run by solar battery
When a large number of solar cells are connected in series it form
a solar battery. Solar battery produces more electricity which is enough to
run, water pump, street-light, etc., They are also used in remote areas where
conventional electricity supply is a problem.
3. Solar cells are used in calculators, electronic watches, radios
and TVs. .
4. Solar cells are superior to other type of cells, because these
are non-polluting and eco-friendly.
5. Solar energy can be stored in Ni-Cd batteries and lead-acid
batteries.
6. Solar cells can be used to drive vehicles.
7. Solar cells, made of silicon, are used as a source of
electricity in space craft and satellites.
8. Write a note on wind energy. (TNV A.U. May 2009, Chen. A.U. June 2009)
(or)
Explain how electric power is generated by using wind energy. (A.U July 2016)
(or)
How is wind energy harnessed? What are its advantages and
limitations. (A.U May 2015)
Moving air is called wind. Energy recovered from the force
of wind is called wind energy. Energy possessed by the wind is due to
its high speed. Kinetic energy of the wind is converted into mechanical energy.
Methods of harnessing wind energy
Wind mill
It is a device used to harness (convert) wind energy into
mechanical energy.
Sequence of energy conversion
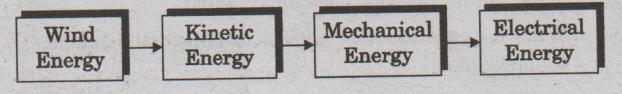
Construction and working of a wind mill
It consists of a wheel containing number of blades. The wheel
rotates about an axle mounted on a pole (Fig.). The wind energy is used to
rotate the wheel. One end of the axle is connected to the armature of a
generator, which rotates between two poles (north and south poles) of a strong
magnet. Another end of the axle is connected to the shaft of the wind mill.
When wind falls on the wheel of a wind mill, it rotates and electric current is
produced. Thus, the kinetic energy of the wind is converted into electric
energy.
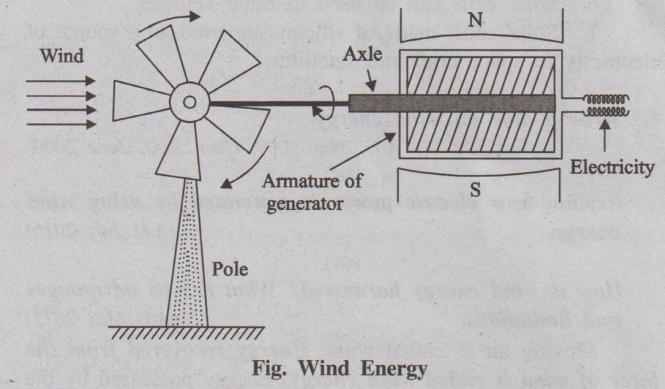
Fig. Wind Energy
Advantages (or) Merits of wind energy
(i) It does not cause any pollution.
(ii) It is very cheap and economic.
(iii) It is renewable.
Disadvantages (or) Demerits of wind energy
1. Wind farms located on the migratory routes of birds will cause
hazards.
2. Wind farms produce unwanted sound.
3. Wind turbines interfere with electromagnetic signals (TV, Radio
signals).
Uses of wind energy
1. Wind energy is used to move the sail boats in lakes, rivers and
seas.
2. It is used to operate water pumps.
3. It is used to run the flour mill to grind the grains.
4. It is also used to produce electricity.
9. Explain various types of highly investigated solar cell materials?
1. Crystalline silicon (c-Si)
Crystalline silicon (C-Si) is the most used (90% of the global PV
market) semiconducting material in solar panels. But, its efficiency is only
30%. So, solar cells with low-cost and high-efficiency materials are emerging.
Examples
(i) III-V multijunction materials: (efficiency > 30%)
(ii) Hybrid tandem III-V/Si solar cells: (efficiency > 30%)
2. Thin films
Due to their narrow design (light weight, flexibility and ease of
installation) second-generation thin-film solar cells are growing as one of the
most promising PV technologies. This films are 350 times smaller light
absorbing layers compared to standard Si-panels.
Examples
(i) Cadmium-telluride (CdTe).
(ii) Amorphous silicon.
3. Perovskite solar cells
Among the next generation solar cells, hybrid metal halide
perovskite solar cells (PSCs), play an important role due to their low price,
thinner design, low temperature processing and excellent light absorption
properties.
Example
Combined perovskite and Si-PV materials shows a record efficiency
of upto 28%.
4. Solar paints
Solar paint is the another transformative technology. These can be
coated over the polymer films.
Examples
(i). Solar paint hydrogen generates energy from photovoltaic water
splitting
(ii) Quantum dots (Photovoltaic paint)
5. Transparent solar windows
They possess highly innovative applications. Their
solar-to-electricity conversion efficiency is 10% more.
6. Thermoradiative PV devices (or) Reverse solar panels
They can generate electricity at night by utilizing the heat
irradiated from the panels to the optically coupled deep space, which serves as
a heat sink.
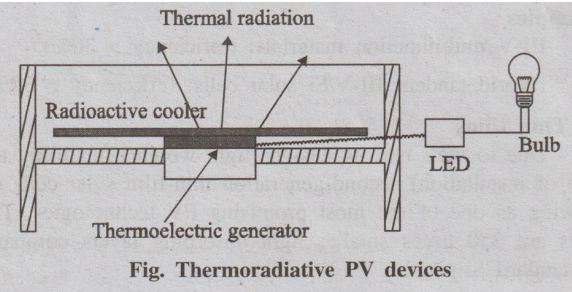
Fig. Thermoradiative PV devices
Engineering Chemistry: Unit V: a. Energy Sources : Tag: Engineering Chemistry : Energy Sources | Engineering Chemistry - Anna University Long Questions and Answers
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
