Engineering Chemistry: Unit V: b. Energy Storage Devices
Anna University Long Questions and Answers
Energy Storage Devices | Engineering Chemistry
Engineering Chemistry : UNIT V : Energy Sources and storage devices : Anna University long Questions & Answers
Unit - v
Chapter 8
Batteries
Anna University Long Questions & Answers
Part - B
1. Explain the construction and working of a lead storage battery.
(Coim A.U Jan 2009) (TNV. AU. May 2009) (Dec 2008, Jan 2013, Dec
2014, Dec 2015)
(or)
What are lead accumulators? Explain the construction and
functioning of a lead accumulator. (A.U. June
2014)
(or)
Describe the construction of lead-acid battery with reaction
occuring during discharging. (A.U June
2016)
A Lead accumulator is a secondary battery, which can operate both
as a voltaic cell and as an electrolytic cell.
Construction
A lead-acid storage battery consists of a number of (3 to 6)
voltaic cells connected in series to get 6 to 12 V battery. In each cell, the
anode is made of lead. The cathode is made of lead dioxide PbO2 or a
grid made of lead, packed with PbO2.. A number of lead plates
(anodes) are connected in parallel and a number of PbO2 plates
(cathodes) are also connected in parallel. Various plates are separated from
the adjacent ones by insulators like rubber or glass fibre. The entire
combinations is then immersed in dil. H2SO4 (38% by mass)
having a density of 1.30 gm/ml. The cell may be represented as;
Pb | PbSO4|| H2SO4 (aq)
| PbO2 | Pb
Working (Discharging)
When the lead-acid storage battery operates, the following
reaction occurs.
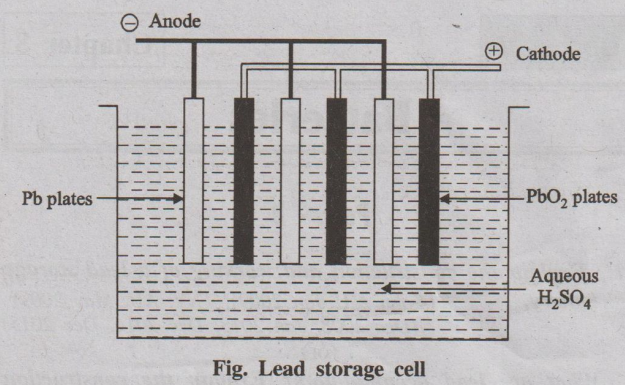
Fig. Lead storage cell
At anode: Lead is oxidized to Pb2+
ions, which further combines with SO2-4 forms
insoluble PbSO4.
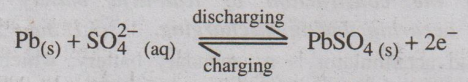
At cathode: PbO2
is reduced to Pb2+ ions, which further combines with SO2-4
forms insoluble PbSO4.
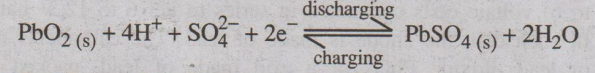
Overall cell reaction during use (discharging):
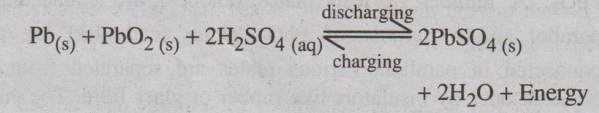
Recharging the Battery
The cell can be charged by passing electric current in the
opposite direction. The electrode reaction gets reversed. As a result, Pb is
deposited on anode and PbO2 on the cathode. The density of H2SO4
also increases.
The net reaction during charging is
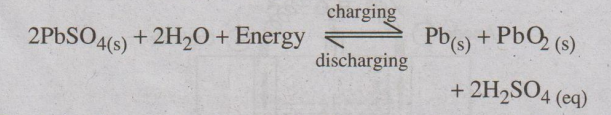
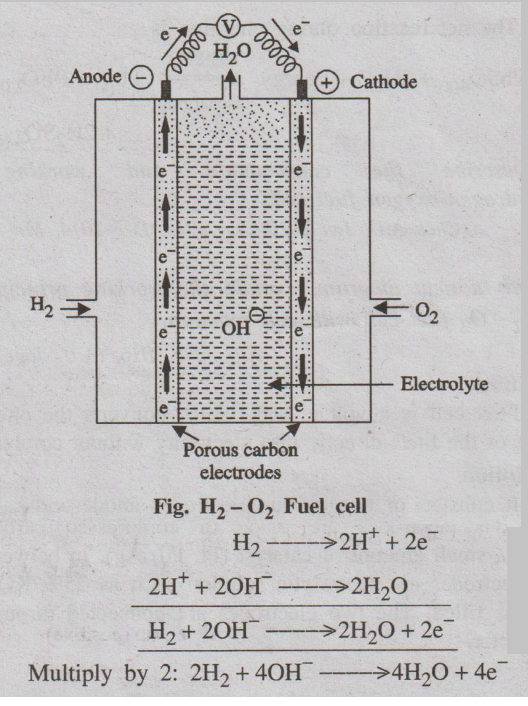
2. Describe the construction and working of hydrogen-oxygen fuel
cell. (Chen A.U. Jan 2010, Jan 2013,
Dec 2014, May 2017)
(or)
With a neat diagram, explain the working principal of H2-O2
fuel cell with cell reaction. (A.U June 2016, (A.U. June 2014)
Definition
Fuel cell is a voltaic cell, which converts the chemical energy of
the fuels directly into electricity without combustion.
Description
It consists of two porous electrodes anode and cathode. These porous
electrodes are made of compressed carbon containing a small amount of catalyst
(Pt, Pd, Ag). In between the two electrodes an electrolytic solution such as
25% KOH or NaOH is filled. The two electrodes are connected through the volt
meter.
Working
Hydrogen (the fuel) is bubbled through the anode compartment,
where it is oxidised. The oxygen (oxidiser) is bubbled through the cathode
compartment, where it is reduced.
Various reactions
At Anode
Hydrogen gas, passed through the anode, is oxidised with the
liberation of electrons which then combine with hydroxide ions to form water.
At cathode
The electrons, produced at the anode, pass through the external
wire to the cathode where it is absorbed by oxygen and water to produce
hydroxide ions.
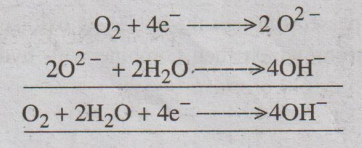
Overall cell reaction
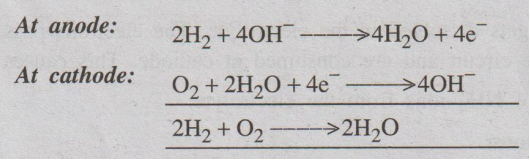
The emf of the cell = 0.8 to 1.0 V
3. Explain the construction, working of Leclanche's Cell
It is a primary cell, which works without fluid component.
Description
A dry cell consists of a zinc cylinder, which acts as anode. This
zinc cylinder is filled with an electrolyte consisting of NH4C1,
ZnCl2 and MnO2 in the form of paste using starch and
water. A carbon rod (graphite), acts as cathode, is immersed in the electrolyte
in the centre of the cell. The zinc cylinder has an outer insulation of
cardboard case. During use, the zinc cylinder gets consumed and at the end, it
will develop holes which are responsible for leakages.
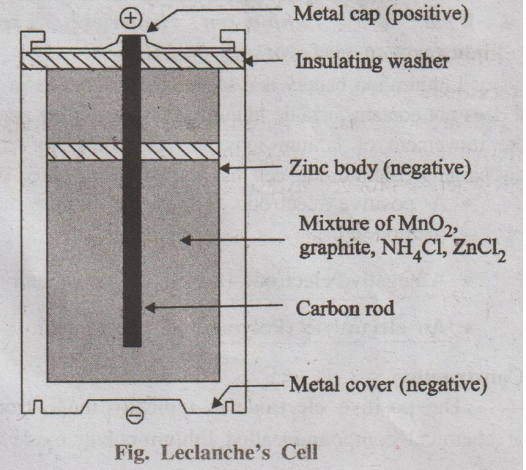
Fig. Leclanche's Cell
Working
When the cell is working, zinc loses electrons and Zn2+
ions gets dissolved in the electrolyte. The electrons pass through the circuit
and are consumed at cathode. This causes discharge of NH+4
ions from the electrolyte.
Cell reactions:
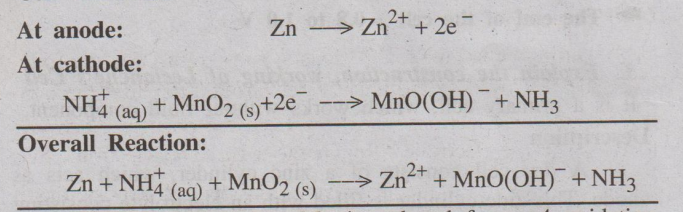
In cathode reaction, Mn is reduced from +4 oxidation state to +3
oxidation state. The liberation of NH3 gas, which disrupts the
current flow, is prevented by a reaction of
NH3 (g) with Zn2+ (from ZnCl2).
ZnCl2 + 2 NH3 → [Zn(NH3)2]
Cl2(s)
The voltage of Leclanche's cell is about 1.5 V.
4. What are Lithium-ion batteries? Explain the construction and
working of LIB
Lithium-ion battery is a secondary battery. As in lithium cell, it
does not contain metallic lithium as anode. As the name suggests, the movement
of lithium ions are responsible for charging and discharging. Lithium-ion cell
has the following three components.
* A positive electrode (Layers of lithium-metal oxide) (cathode)
* A negative electrode (Layers of porous carbon) (anode)
* An electrolyte (Polymer gel) (separator)
Construction
The positive electrode is typically made from a layers of chemical
compound called lithium-cobalt oxide (LiCoO2).
The negative electrode is made from layers of porous carbon (C)
(graphite).
Both the electrodes are dipped in a polymer gel electrolyte (organic
solvent) and separated by a separator, which is a perforated plastic and allows
the Litions to pass through.
Working
Charging
During charging, Li+ ions flow from the positive
electrode (LiCoO2) to the negative electrode (graphite) through the
electrolyte. Electrons also flow from the positive electrode to the negative
electrode through the wire. The electrons and Li+ ions combine at
the negative electrode and deposit there as Li. -
LiCoO2 + C → Li 1- x CoO2 + CLix
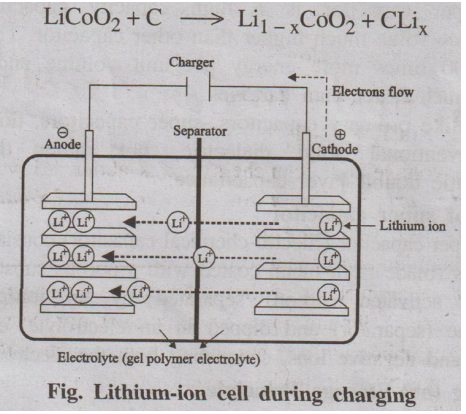
Fig. Lithium-ion cell during charging
Discharging
During discharging, the Li+ ions flow back through the
electrolyte from negative electrode to the positive electrode. Electrons flow
from the negative electrode to the positive electrode through the wire. The Li+
ions and electrons combine at the positive electrode and deposit there as Li+.
Li 1- x CoO2 → LiCoO2 + C
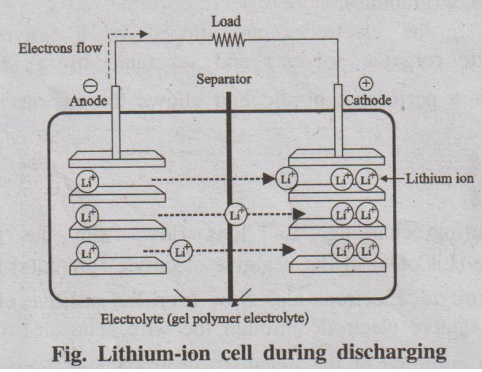
Fig. Lithium-ion cell during discharging
5. Write notes an Super Capacitor
Super capacitor is a high capacity capacitor with capacitance
value much higher than other capacitor. They store 10 to 100 times more energy
per unit volume and deliver charge much faster than batteries.
Unlike ordinary capacitors, super capacitors, do not use the
conventional solid dielectric, but rather they use electrostatic double-layer
capacitance.
Design of super capacitor
Super capacitor (Electro-chemical capacitor) consists of two
electrodes (made from metal coated with a porous substance like powdery
activated carbon separated by an ion-permeable membrane (separator) and dipped
in an electrolyte, containing positive and negative ions, connecting both the
electrodes.
Working (or) Storage Principle
When the electrodes are connected to the power source, ions in the
electrolyte form electric double layers (Helmholtz electrical double layer) of
opposite polarity to the electrodes polarity, creating an electric field
between them.
. For example, positively polarized electrodes will have a layer
of negative ions at the electrode/electrolyte interface.
Similarly negatively polarised electrodes will have a layer of
positive ions at the electrode/electrolyte interface.
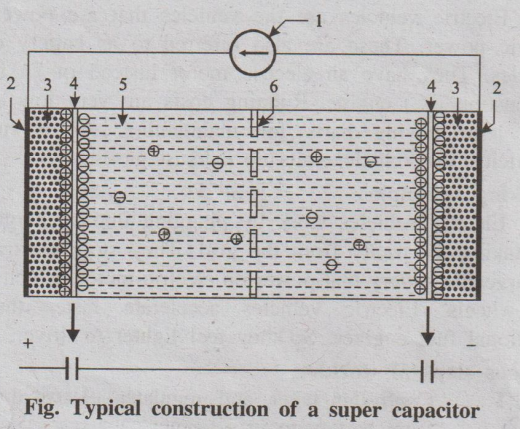
Fig. Typical construction of a super capacitor
1. Power source
2. Collector
3. Polarized electrode
4. Helmholtz double layer
5. Electrolyte having positive and negative ions
6. Separator
This electric field polarizes the dielectric, so its molecules
lineup in the opposite direction to the field and reduce its strength. It means
that it stores more electrical energy at an electrode-electrolyte interface.
Advantages
1. It is highly safe
2. Its life time is very high (10 to 20 years)
3. It can be cycled millions of time
Disadvantages
1. Cost per watt is high.
2. It cannot be used as source for continuous power supply
3. If higher voltage is required, the cells must be connected in
series.
Applications
Super capacitors are used in many power management applications
like,
1. Voltage stabilization in start/stop system,
2. Energy harvesting,
3. Kitchen appliances
6. What are electric vehicles? Explain their working principle and
advantages and disadvantages?
Electric vehicles are the vehicles that are powered on electric
power. These are also referred to as battery electric vehicles. They have an
electric motor instead of an internal combustion (IC) engine. Running costs are
very low as they have less moving parts for maintaining. As it runs on
electricity, the vehicle emits no exhaust gases.
Working Principle
Electric vehicles work by plugging into a charge point and taking
electricity from the grid. They store electricity in rechargeable battery that
power on electric motor, which rotates the wheels. Electric vehicles accelerate
faster than the traditional fuel engines. So they feel lighter to drive.
Various steps of working
Step I Controller takes and
regulates electrical energy from battery to inverter.
Step II The inverter then sends a
certain amount of electrical energy to the motor.
Step III The motor converts
electrical energy into mechanical energy (rotation).
Step IV Rotation of the motor rotor
rotates the transmission, so the wheels turn and then the vehicle moves.
Step V When the brakes are
pressed, the motor becomes an alternator and produces power, which is sent back
to the battery.
Advantages
(i) Electric cars are energy efficient
(ii) It reduces emission.
(iii) Its performance is high and has low maintenance.
(iv) It can be fuelled for very low price.
(v) It is more convenient and easy to recharge.
Disadvantages
(i) - Electric cars can travel less distance.
(ii) It takes longer time to refuel (recharge)
(iii) These are more expensive and battery packs may need to
be replaced.
(iv) Electric fuelling stations are still in the developing
stages.
(v) Initial investment is very high.
7. What is microbial fuel cell? Explain its principle and working
with neat diagram.
Microbial fuel cell is a device that converts chemical energy to
electrical energy by the action of micro-organisms under anaerobic conditions.
Bioelectricity is generated by the oxidation of organic waste and
renewable biomass using bacteria.
Construction (or) Principle
MFCs are type of electrochemical cells, constructed using either
bioanode and (or) a biocathode. A membrane separates the compartments of the
anode (where oxidation occurs) and the cathode (where reduction occurs). The
electrons produced during oxidation are transferred directly to the cathode.
The charge balance of the system is maintained by the ionic movement inside the
cell.
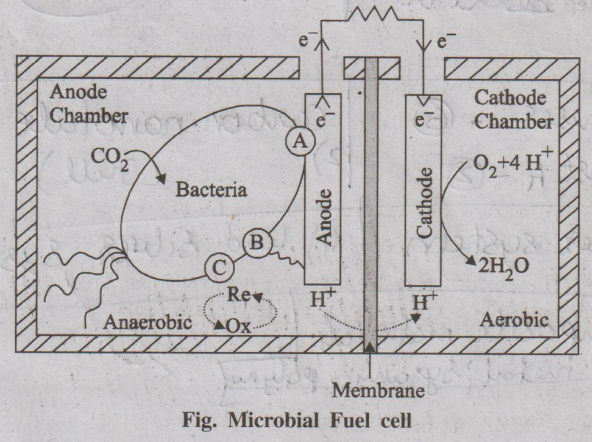
Fig. Microbial Fuel cell
Organic electron donors, that is oxidized to produce CO2,
protons and electrons, are used in most MFCs. The cathode reaction uses a
variety of electron acceptors, mostly oxygen (O2).
Working
When both the electrodes are connected, anode oxidation occurs on organic
waste (biomass) and electrons released from the process are transferred to the
anode. The electrons, transfered to the anode, can be accomplished by the
electron mediators.
From the anode these electrons are directed to the cathode across
an external circuit. For every electron, conducted, a proton is transported
across the membrane to the cathode. Finally oxygen present at the cathode
recombines with hydrogen and electron to produce water.
Engineering Chemistry: Unit V: b. Energy Storage Devices : Tag: Engineering Chemistry : Energy Storage Devices | Engineering Chemistry - Anna University Long Questions and Answers
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
