Engineering Chemistry: Unit III: a. Phase Rule
Binary Alloy System (or) The Simple Eutectic System
Lead-Silver system | Phase Rule
1. Curve AO, 2. Curve BO, 3. Point 'O' (Eutectic point), 4. Areas.
BINARY ALLOY SYSTEM (Or) THE SIMPLE EUTECTIC SYSTEM
1. The Lead-Silver system
Since the system is studied at constant pressure, the vapour phase
is ignored and the condensed phase rule is used.
F' = C – P + 1
The phase diagram of lead-silver system is shown in Fig. 3.5. It
contains lines, areas and the eutectic point.
1. Curve AO
The curve AO is known as freezing point curve of silver. Point A
is the melting point of pure Ag (961°C). The curve AO shows the melting point
depression of Ag by the successive addition of Pb. Along this curve AO, solid
Ag and the melt are in equilibrium.
Solid Ag ⇌ Melt
According to reduced phase rule equation.
F' = C – P + 1; F' = 2 - 2 + 1; F' = 1
The system is univariant.
2.
Curve BO
The curve BO is known as freezing point curve of lead. Point B is
the melting point of pure lead (327°C). The curve BO shows the melting point
depression of ‘Pb' by the successive addition of ‘Ag?. Along this curve ‘BO',
solid ‘Pb' and the melt are in equilibrium.
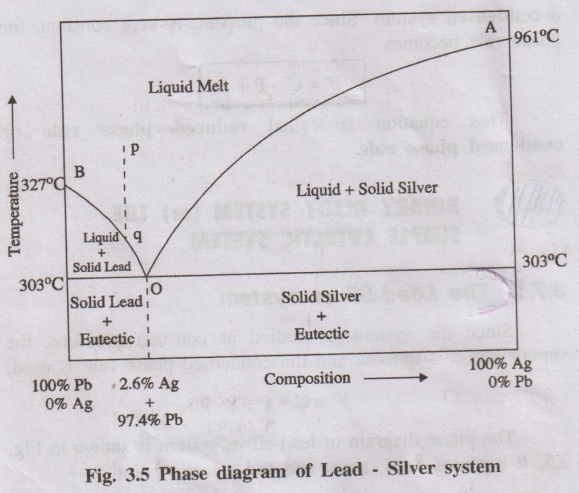
Fig. 3.5 Phase diagram of Lead - Silver system
Solid Pb ⇌ Melt
According to reduced phase rule equation.
F' = C – P + 1; F' = 2 - 2 + 1; F' = 1
The system is univariant
3. Point 'O' (Eutectic point)
The curves AO and BO meet at point “O’at a temperature of 303°C,
where three phases (solid Ag, solid Pb and their liquid melt) are in
equilibrium.
Solid Pb + Solid Ag ⇌ Melt
According to reduced phase rule equation.
F' = C – P + 1; F' = 2 – 3 + 1; F' = 0
The system is non-variant.
The point 'O' is called eutectic point or eutectic temperature and
its corresponding composition, 97.4%Pb + 2.6%Ag, is called eutectic composition.
Below this point the eutectic compound and the metal solidify.
4. Areas
The area above the line AOB has a single phase (molten Pb + Ag).
According to reduced phase rule equation.
F' = C – P + 1; F' = 2 - 1 + 1; F' = 2
The system is bivariant.
Both the temperature and composition have to be specified to
define the system completely.
The area below the line AO (solid Ag + liquid melt), below the
line BO (solid Pb + liquid melt) and below the point 'O' (Eutectic compound +
solid Ag or solid Pb) have two phases and hence the system is univariant
F' = C - P + 1; F' = 2 - 2 + 1; F' = 1.
Application of Pattinson's process for the desilverisation of
Argentiferous lead
The argentiferous lead, consisting of a very small amount of
silver (say 0.1%), is heated to a temperature above its melting point, so that
the system consisting of only the liquid phase represented by the point 'p' in
the Figure 3.5. It is then allowed to cool. The temperature falls down along
the line ‘pq'. As soon as the point 'q' is reached, Pb is crystallised out and
the solution will contain relatively increasing amount of ‘Ag'. On further
cooling, more and more ‘Pb' is separated along the line ‘BO'the melt continues
to be richer and richer in silver until the point O is reached, where the percentage
of Ag rises to 2.6%.
Thus, the process of raising the relative proportion of Ag in the
alloy is known as Pattinson's process.
Uses of Eutectic system
1. Suitable alloy composition can be predicted with the help of
eutectic systems.
2. Eutectic systems are used in preparing solders, used for
joining two metal pieces together.
Engineering Chemistry: Unit III: a. Phase Rule : Tag: Engineering Chemistry : Lead-Silver system | Phase Rule - Binary Alloy System (or) The Simple Eutectic System
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
