Engineering Chemistry: Unit I: Water and its Treatment
Boiler Troubles (or) Boiler Feed Water
The water fed into the boiler for the production of steam is called boiler feed water. Boiler feed water should be free from turbidity, oil, dissolved gases, alkali and hardness causing substances.
BOILER TROUBLES (OR)
BOILER FEED WATER
The water fed into the boiler for the production of steam is
called boiler feed water. Boiler feed water should be free from turbidity, oil,
dissolved gases, alkali and hardness causing substances. If hard water obtained
from natural sources is fed directly into the boilers, the following troubles
may arise.
Boiler troubles (or) disadvantages of using hardwater in boilers
1. Formation of Scales and sludges in boilers.
2. Priming and foaming (carry over).
3. Caustic embrittlement.
4. Boiler corrosion.
1. Formation of Scales and Sludges in boilers
When water is continuously converted into steam in , boilers (or)
heat exchangers, the concentration of dissolved salts in water increases
progressively. When the concentration of the salts reaches their saturation
point, they are thrown out in the form of precipitates on the inner walls of
the boilers (or) heat exchangers. The least soluble one gets precipitated
first.
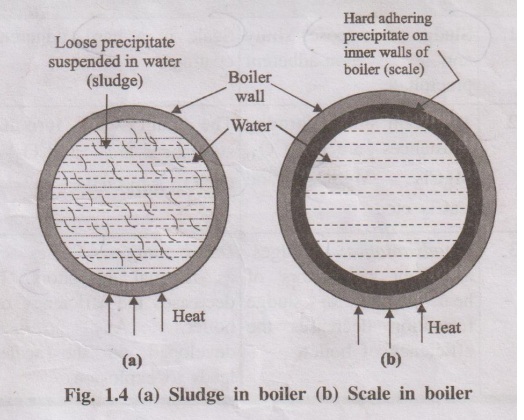
1. Sludge (Loose deposit)
If the precipitate is loose and slimy it is called sludge. Sludges
are formed by the substances like MgCl2, MgCO3, MgSO4
and CaCl2 . They have greater solubilities in hot water than cold
water.
2. Scale (Hard deposit)
On the other hand, if the precipitate forms hard and adherent
coating on the inner walls of the boiler, it is called scale. Scales are formed
by substances like Ca(HCO3)2, CaSO4 and Mg(OH)2
Table 1.1 Comparison of Scales and Sludges
Sludge
1. Sludge is a loose slimy
and non-adherent precipitate.
2. The main sludge forming
substance are MgCO3 , MgCl2, MgCO3, and CaCl2
etc,.
3. Disadvantages :
Sludges are poor conductors of heat. Excess of sludge formation decreases the
efficiency of boiler.
4. Prevention
(i) Sludge formation can be
prevented by using softened water.
(ii) Sludges can also be
removed by “blow-down operation”.
(iii) Blow-down operation is
a prpcess of removing a portion of concentrated water frequently from the
bolier during steam production.
Scale
1. Scale is a hard, adherent
coating.
2. The main scale forming
substances are Ca(HCO3)2, CaSO4, Mg(OH)2.
3. Disadvantages : Scale
act as thermal insulators. It decreases the efficiency of boliler. Any crack
developed on the scale, leads to explosion.
4. Prevention
(i) Scale formation can be
prevented by dissolving using acids like HCl, H2SO4.
(ii) Scale formation can be
removed by
(a) External treatment.
(b) Internal treatment
(iii) They can also be
removed by applying thermal shocks, scrapers, wire brush, etc,.
Disadvantages of Scale Formation
1. Wastage of fuels
Scales have low thermal conductivity, so the heat transfer from
boiler to inside water is not efficient. In order to provide steady supply of
heat to water, overheating is done and this causes wastage of fuel. The wastage
of fuel depends on the thickness and nature of the scale, which is shown in the
table.

2. Decrease in efficiency
Scales sometimes deposit in the valves and condensers of the
boiler and choke. This results in decrease efficiency of the boiler.
3. Boiler explosion
Sometimes due to over heating the thick scales may crack and
causes sudden contact of high heated boiler material with water. This causes
formation of a large amount of steam and high pressure is developed which may
lead to explosion.
Prevention of scale formation
1. At the initial stage, scales can be removed using scraper, wire
brush etc.
2. If scales are brittle, they can be removed by thermal shocks.
3. By using suitable chemicals like dil. acids (for CaCO3
scale), EDTA (for CaSO4 scale) with which they form suitable
complexes.
4. If the scales are loosely adhering, they can be removed by
frequent blow down operation
2. Priming and Foaming (carry over)
During the production of steam in the boiler, due toʻrapid
boiling, some droplets of liquid water are carried along with steam. Steam
containing droplets of liquid water is called wet steam. These droplets of
liquid water carry with them some dissolved salts and suspended impurities,
This phenomenon is called carry over. It occurs due to printing and foaming.
1. Priming
Priming is the process of production of wet steam. Priming is
caused by
(i) High steam velocity.
(ii) Very high water level in the boiler.
(iii) Sudden boiling of water.
(iv) Very poor boiler design.
Prevention
Priming can be controlled by
(i) Controlling the velocity of steam.
(ii) Keeping the water level lower.
(iii) Good boiler design.
(iv) Using treated water.
2. Foaming
The formation of_stable bubbles above the surface of water is
called foaming. These bubbles are carried over by steam leading to excessive
priming.
Foaming is caused by the
(i) presence of oil, and grease,
(ii) presence of finely divided particles.
Prevention
Foaming can be prevented by
(i) adding coagulants like sodium aluminate, aluminium hydroxide,
(ii) adding anti-foaming agents like synthetic polyamides.
3. Caustic Embrittlement (Intercrystalline Cracking)
Caustic embrittlement means intercrystalline cracking of boiler
metal.
Boiler water usually contains a small proportion of Na2CO3.
In high pressure boilers this Na2CO3 undergoes
decomposition to give NaOH.
Na2CO3 + H2O → 2NaOH + CO2
This NaOH flows into the minute hair cracks and crevices, usually
present on the boiler material, by capillary action and dissolves the
surrounding area of iron as sodium ferroate.
Fe + 2NaOH → Na2FeO2 + H2 ↑
This causes brittlement of boiler parts, particularly stressed
parts like bends, joints, rivets, etc., causing even failure of the boiler.
Prevention
Caustic embrittlement can be prevented by
(i) using sodium phosphate as softening agent instead of sodium
carbonate.
(ii) by adding tannin, lignin to the boiler water, which blocks
the hair cracks.
4. Boiler corrosion
Corrosion in boilers is due to the presence of
1. dissolved oxygen.
2. dissolved carbon dioxide.
3. dissolved salts.
1. Dissolved oxygen
Dissolved oxygen in water is mainly responsible for the corrosion
of boiler. The dissolved oxygen in water attacks the boiler material at higher
temperature.
4Fe + 6H2O + 3O2 → 4Fe(OH)3 ↓
Removal of dissolved
oxygen
Dissolved oxygen can be removed by chemical (or) mechanical
methods.
(a) Chemical method
Sodium sulphite, hydrazine are some of the chemicals used for
removing dissolved oxygen.
2Na2SO3 + O2 → 2Na2SO4
N2H4 + O2 → N2 + 2H2O
Hydrazine is found to be an ideal compound for removing dissolved
oxygen in the water, since the products are water and inert N2 gas.
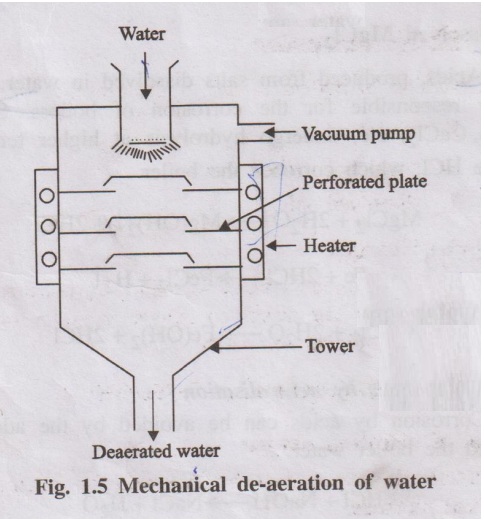
(b) Mechanical
de-aeration
Dissolved oxygen can also be removed from water by mechanical
deaeration (Fig. 1.5).
In this process, water is allowed to fall slowly on the perforated
plates fitted inside the tower. The sides of the tower are heated, and a vacuum
pump is also attached to it. The high temperature and low pressure produced
inside the tower reduce the dissolved oxygen content of the water.
2. Dissolved carbon dioxide
Dissolved carbon dioxide in water produces carbonic acid, which is
acidic and corrosive in nature
CO2+ H2O → H2CO3
Carbon dioxide gas is also produced from the decomposition of
bicarbonate salts present in water.
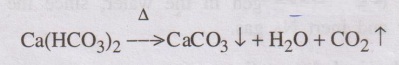
Removal of dissolved
Carbon dioxide
(a) Carbon dioxide can be removed from water by adding a
calculated amount of NH4OH into water.
2NH4OH+CO2 → (NH4)2CO3
+ H2O
(b) Carbon dioxide along with oxygen can also be removed
mechanically by de-aeration method.
3. Dissolved MgCl2
Acids, produced from salts dissolved in water, are also mainly
responsible for the corrosion of boilers. Salts like MgCl2, CaCl2,
etc, undergo hydrolysis at higher temperature to give HCl, which corrodes the
boiler.
MgCl2 + 2H2O → Mg(OH)2 ↓+ 2HCI
Fe + 2HCl → FeCl2 + H2↓
FeCl2 + 2H2 O → Fe(OH)2 + 2HCI
Removal of acids by
neutralisation
Corrosion by acids can be avoided by the addition of alkali to the
boiler water.
HCl + NaOH → NaCl + H2O
Engineering Chemistry: Unit I: Water and its Treatment : Tag: Engineering Chemistry : - Boiler Troubles (or) Boiler Feed Water
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
