Engineering Chemistry: Unit I: Water and its Treatment
Desalination of Brackish Water
Grade, RO Process, Advantages
The process of removing common salt (sodium chloride) from the water is known as desalination.
DESALINATION OF
BRACKISH WATER
The process of removing common salt (sodium chloride) from the
water is known as desalination. The water containing dissolved salts with a
peculiar salty (or) brackish taste is called brackish water.
Depending upon the quantity of dissolved solids, water is graded
as
1. Fresh water - Contains
< 1000 ppm of dissolved solids.
2. Brackish water - Contains
> 1000 but < 35,000 ppm of dissolved solids.
3. Sea water - Contains
> 35,000 ppm of dissolved solids.
Sea water and brackish water can be made available as drinking
water through desalination process. Desalination is carried out by reverse
osmosis.
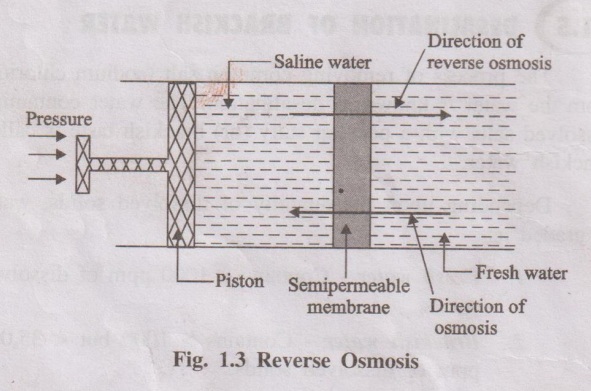
1. Reverse Osmosis (RO) Com
When two solutions of different concentrations are separated by a
semi-permeable membrane, solvent (water) flows from a region of lower
concentration to higher concentration. This process is called osmosis. The
driving force in this phenomenon is called osmotic pressure.
If a hydrostatic pressure in excess of osmotic pressure is applied
on the higher concentration side, the solvent flow is reversed i.e., solvent
flows from higher concentration to lower concentration. This process is called
reverse osmosis (Fig. 1.3) Thus, in the process of reverse osmosis pure water
is separated from salt water. This process is also known as super-filtration.
The membranes used are cellulose acetate, cellulose butyrate, etc.
Advantages
(i) The life time of the membrane is high, and it can be replaced
within few minutes.
(ii) It removes ionic as well as non-ionic, colloidal impurities.
(iii) Due to low capital cost, simplicity, low operating, this
process is used for converting sea water into drinking water.
Engineering Chemistry: Unit I: Water and its Treatment : Tag: Engineering Chemistry : Grade, RO Process, Advantages - Desalination of Brackish Water
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
