Engineering Chemistry: Unit V: b. Energy Storage Devices
Fuel Cells
Definition, Working Principle, Cell reactions, Applications, Advantages, Limitations, Disadvantages
Fuel cell is a voltaic cell, which converts the chemical energy of the fuels directly into electricity without combustion.
FUEL CELLS
Definition
Fuel cell is a voltaic cell, which converts the chemical energy of
the fuels directly into electricity without combustion.
Fuel cell converts the energy of the fuel directly into
electricity. In these cells, the reactants, products and electrolytes pass
through the cell.
Fuel + Oxygen → Oxidation products + Electricity .
Example
Hydrogen-oxygen fuel cell.
Fuel Battery
When a large number of fuel cells are connected in series, it
forms fuel battery.
1. Hydrogen-Oxygen fuel cell
Hydrogen-oxygen fuel cell is the simplest and most successful fuel
cell, in which the fuel-hydrogen and the oxidiser-oxygen and the liquid
electrolyte are continuously passed through the cell.
Description
It consists of two porous electrodes anode and cathode. These
porous electrodes are made of compressed carbon containing a small amount of
catalyst (Pt, Pd, Ag). In between the two electrodes an electrolytic solution
such as 25% KOH or NaOH is filled. The two electrodes are connected through the
volt meter.
Working
Hydrogen (the fuel) is bubbled through the anode compartment,
where it is oxidised. The oxygen (oxidiser) is bubbled through the cathode
compartment, where it is reduced.
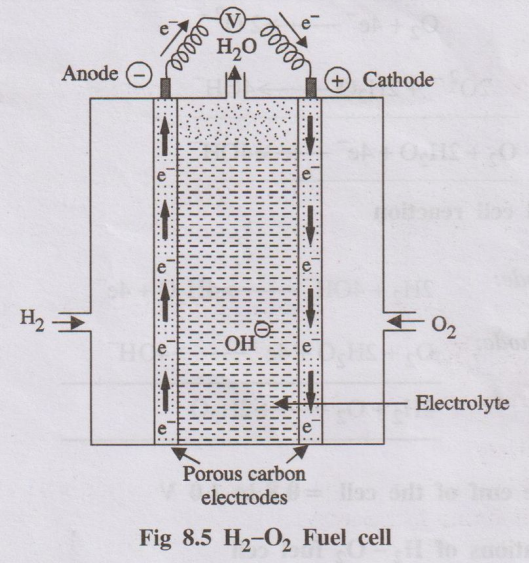
Fig 8.5 H2-02 Fuel cell
Various reactions
At Anode
Hydrogen gås, passed through the anode, is oxidised with the
liberation of electrons which then combine with hydroxide ions to form water.
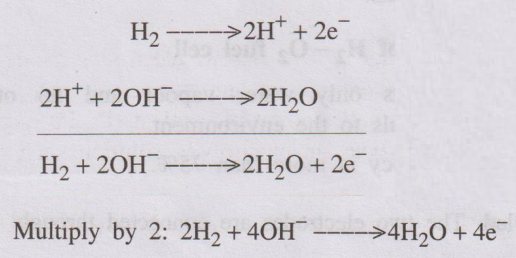
At cathode
The electrons, produced at the anode, pass through the external
wire to the cathode where it is absorbed by oxygen and water to produce
hydroxide ions.
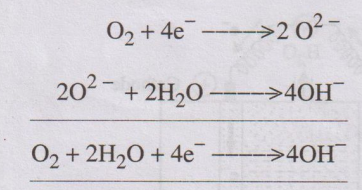
Overall cell reaction
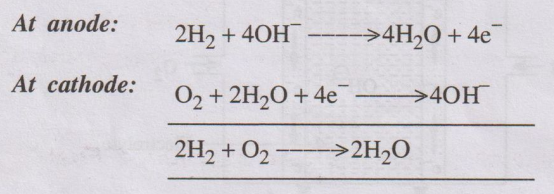
The emf of the cell = 0.8 to 1.0 V
Applications of H2 - O2 fuel cell
1. H2 - O2 fuel cells are used as auxiliary
energy source in space vehicles, submarines or other military-vehicles.
2. In case of H2 - O2 fuel cells, the
product of water is proved to be a valuable source of fresh water by the
astronauts.
Advantages of H2 - O2 fuel cell
(i) It emits only water vapour and no other harmful chemicals to
the environment.
(ii) Efficiency is more than 75%.
(iii) As hydrogen is the lightest element, it can be transported easily
from one place to another.
(iv) Hydrogen, as a fuel, can replace the use of batteries.
(v) It causes less noise pollution.
Limitations (or) Disadvantages of H2 - O2
fuel cell
1. Hydrogen gas is explosive.
2. It is very expensive to be carried out.
3. As hydrogen is a gas, it is difficult to compress into liquid
form.
4. Hydrogen is not present as it is, but always present in
combined form with either oxygen or some other element, so it must be separated
first.
5. While using H2 - O2 fuel cell in
an automobile, a high pressure must be created inside the engine, which is
risky.
2. Advantage and Disadvantages of Fuel cells
Advantages of Fuel cells
1. Fuel cells are efficient (75%) and take less time for operation.
2. It is pollution free technique.
3. It produces electric current directly from the reaction o a
fuel and an oxidiser.
4. It produces drinking water.
Disadvantages of Fuel cells
1. Fuel cells can not store electric energy as other cells do.
2. Electrodes are expensive and short lived.
3. Storage and handling of hydrogen gas is dangerous.
3. Microbial Fuel Cells (MFCs)
Microbial fuel cell is a device that converts chemical energy to
electrical energy by the action of micro-organisms under anaerobic conditions.
Bioelectricity is generated by the oxidation of organic waste and
renewable biomass using bacteria.
Construction (or) Principle
MFCs are type of electrochemical cells, constructed using either
bioanode and (or) a biocathode. A membrane separates the compartments of the anode
(where oxidation occurs) and the cathode (where reduction occurs). The
electrons produced during oxidation are transferred directly to the cathode.
The charge balance of the system is maintained by the ionic movement inside the
cell.
Organic electron donors, that is oxidized to produce CO2,
protons and electrons, are used in most MFCs. The cathode reaction uses a.
variety of electron acceptors, mostly oxygen (O2).
Components
typical MFC consists of the following two compartments.
(i) Anodic compartment
(ii) Cathodic compartment
(i) Anodic compartment
It consists of microbes suspended under anaerobic conditions in
the anolyte.
(ii) Cathodic compartment
It consists of electron acceptor (oxygen)
Permeable membrane
Anionic and cationic compartments are separated by a selectively
permeable, cation-specific membrane.
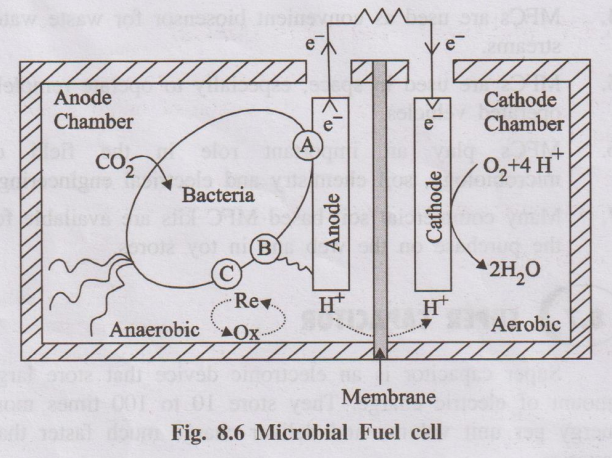
Fig. 8.6 Microbial Fuel cell
Working
When both the electrodes are connected, anode oxidation occurs on
organic waste (biomass) and electrons released from the process are transferred
to the anode. The electrons, transfered to the anode, can be accomplished by
the electron mediators.
From the anode these electrons are directed to the cathode across
an external circuit. For every electron, conducted, a proton is transported
across the membrane to the cathode. Finally oxygen present at the cathode
recombines with hydrogen and electron to produce water.
Applications
1. In waste water treatment, MFCs, generate less excess sludge as
compared to the aerobic treatment process.
2. MFCs can be used in river and deep-water environments, where it
is difficult to use batteries.
3. MFCs are used to convert carbon rich wastewater into methane
gas.
4. MFCs are used as convenient biosensor for waste water streams.
5. MFCs are used in space, especially to operate remotely operated
vehicles.
6. MFCs play an important role in the field of microbiology, soil
chemistry and electrical engineering.
7. Many commercial soil based MFC kits are available for the
purchase on the web and in toy stores.
Engineering Chemistry: Unit V: b. Energy Storage Devices : Tag: Engineering Chemistry : Definition, Working Principle, Cell reactions, Applications, Advantages, Limitations, Disadvantages - Fuel Cells
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
