Engineering Chemistry: Unit V: b. Energy Storage Devices
Important Primary Battery: Dry Cell (or) Leclanche's Cell
Description, Diagram, Construction, Working Principle, Cell reactions, Disadvantages, Uses
It is a primary cell, which works without fluid component.
IMPORTANT PRIMARY BATTERY
1. Dry Cell (or) Leclanche's Cell
It is a primary cell, which works without fluid component.
Description
A dry cell consists of a zinc cylinder, which acts as anode. This
zinc cylinder is filled with an electrolyte consisting of NH4C1,
ZnCl2 and MnO2 in the form of paste using starch and
water. A carbon rod (graphite), acts as cathode, is immersed in the electrolyte
in the centre of the cell. The zinc cylinder has an outer insulation of
cardboard case. During use, the zinc cylinder gets consumed and at the end, it
will develop holes which are responsible for leakages.
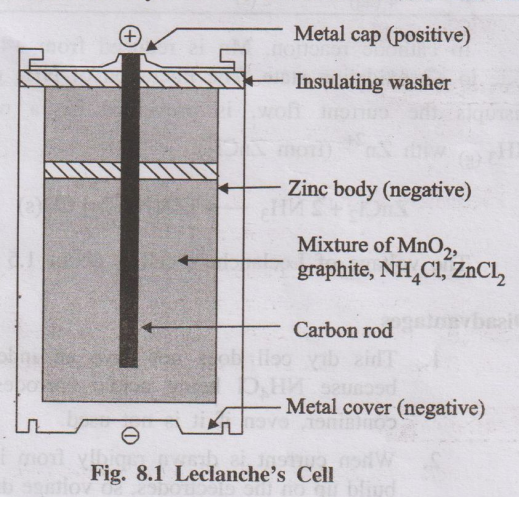
Fig. 8.1 Leclanche's Cell
Working
When the cell is working, zinc loses electrons and Zn2+ ions
gets dissolved in the electrolyte. The electrons pass through the circuit and
are consumed at cathode. This causes discharge of NH+4
ions from the electrolyte.
Cell reactions:
At anode:
Zn → Zn2+ + 2e-
At cathode:
NH+4 (aq) + MnO2 (s)+
2e- → MnO(OH) - + NH3
Overall Reaction:
Zn + NH+4 (aq) + MnO2 (s)
→ Zn2+ + MnO(OH) - + NH3
In cathode reaction, Mn is reduced from +4 oxidation state to +3
oxidation state. The liberation of NH3 gas, which disrupts the
current flow, is prevented by a reaction of NH3 (g) with Zn2+
(from ZnCl2).
ZnCl2 + 2 NH3 → [Zn(NH3)2] C12(s)
The voltage of Leclanche's cell is about 1.5 V.
Disadvantages
1. This dry cell does not have an indefinite life, because NH4Cl
being acidic corrodes the zinc container, even if it is not used.
2. When current is drawn rapidly from it, products build up on the
electrodes, so voltage drop occurs.
Uses:
It is used in transistor radios, calculators, Flash lights,
torches etc.,
Engineering Chemistry: Unit V: b. Energy Storage Devices : Tag: Engineering Chemistry : Description, Diagram, Construction, Working Principle, Cell reactions, Disadvantages, Uses - Important Primary Battery: Dry Cell (or) Leclanche's Cell
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
