Engineering Chemistry: Unit V: b. Energy Storage Devices
Important Secondary Batteries: Lead - Acid Storage Cell, Lithium-ion battery (LIB) (or) Lithium-ion cell
Description, Diagram, Construction, Working Principle, Cell reactions, Advantages, Disadvantages, Uses
A lead acid storage cell is a secondary battery, which can operate both as a voltaic cell and as an electrolytic cell.
IMPORTANT SECONDARY BATTERIES
1. Lead - Acid Storage Cell
Storage cell
A lead acid storage cell is a secondary battery, which can operate
both as a voltaic cell and as an electrolytic cell. When it acts as a voltaic
cell, it supplies electrical energy and becomes “run down”. When it is
recharged, the cell operates as an electrolytic cell.
Description
A lead-acid storage battery consists of a number of (3 to 6)
voltaic cells connected in series to get 6 to 12 V battery. In each cell, the
anode is made of lead. The cathode is made of lead dioxide PbO2 or a
grid made of lead, packed with PbO2. A number of lead plates
(anodes) are connected in parallel and a number of PbO2 plates
(cathodes) are also connected in parallel.) Various plates are separated from
the adjacent one by insulators like rubber or glass fibre. The entire
combinations is then immersed in dil. H2SO4 (38% by mass)
having a density of 1.30 gm/ml.
The cell may be represented as;
Pb / PbSO4 // H2SO4 (aq) / PbO2 / Pb
Working (Discharging)
When the lead-acid storage battery operates, the following
reaction occurs.
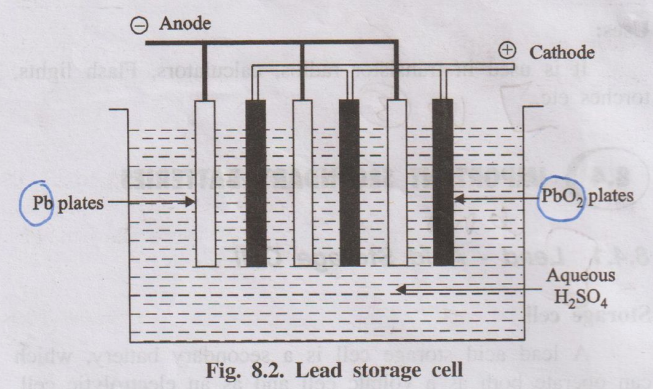
Fig. 8.2. Lead storage cell
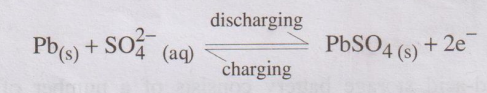
At anode: Lead is oxidized to Pb2+
ions, which further combines with SO2-4 forms
insoluble PbSO4.
At cathode: PbO2
is reduced to Pb2+ ions, which further combines with SO2-4
forms insoluble PbSO4.
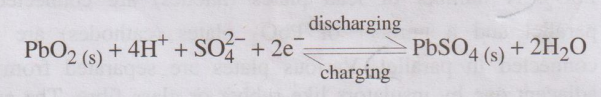
Overall cell reaction during use (discharging):
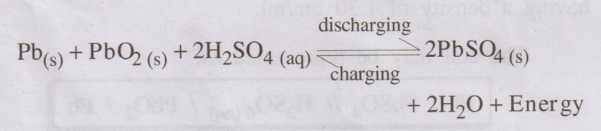
From the above cell reactions it is clear that, PbSO4 is
precipitated at both the electrodes and H2SO4 is used up.
As a result, the concentration of H2SO4 decreases and
hence the density of H2SO4 falls below 1.2 gm/ml. So the
battery needs recharging
Recharging the Battery
The cell can be charged by
passing electric current in the opposite direction. The electrode reaction gets
reversed. As a result, Pb is deposited on anode and PbO2 on the
cathode. The density of H2SO4 also increases.
The net reaction during charging is
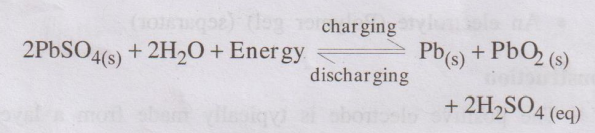
Advantages of lead-acid batteries
(i) It is made easily.
(ii) It produces very high current.
(iii) The self-discharging rate is low when compared to other rechargeable
batteries.
(iv) It also acts effectively at low temperature.
Disadvantages of lead-acid batteries
(i) Recycling of this battery causes environmental hazards.
(ii) Mechanical strain and normal bumping reduces battery capacity.
Uses
1. Lead storage cell is used to supply current mainly in automobiles
such as cars, buses, trucks, etc.,
2. It is also used in gas engine ignition, telephone exchanges,
hospitals, power stations, etc.,
2. Lithium-ion battery (LIB) (or) Lithium-ion cell
Lithium-ion battery is a secondary battery. As in lithium cell, it
does not contain metallic lithium as anode. As the name suggests, the movement
of lithium ions are responsible for charging and discharging. Lithium-ion cell
has the following three components.
• A positive electrode (Layers of lithium-metal oxide) (cathode)
• A negative electrode (Layers of porous carbon) (anode)
• An electrolyte (Polymer gel) (separator)
Construction
The positive electrode is typically made from a layers of chemical
compound called lithium-cobalt oxide (LiCoO2).
The negative electrode is made from layers of porous carbon (C)
(graphite).
Both the electrodes are dipped in a polymer gel electrolyte
(organic solvent) and separated by a separator, which is a perforated plastic
and allows the Litions to pass through
Working
Charging
During charging, Li+ ions flow from the positive
electrode (LiCoO2) to the negative electrode (graphite) through the
electrolyte. Electrons also flow from the positive electrode to the negative
electrode through the wire. The electrons and Li+ ions combine at
the negative electrode and deposit there as Li.
LiCoO2 + C → Li1 - x CoO2
+ CLix
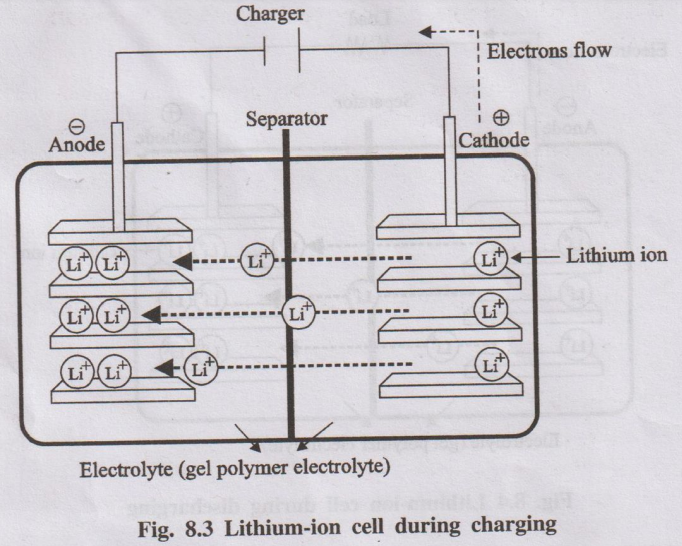
Fig. 8.3 Lithium-ion cell during charging
Discharging
During discharging, the Li+ ions flow back through the
electrolyte from negative electrode to the positive electrode. Electrons flow
from the negative electrode to the positive
electrode through the wire. The Li+ ions and electrons
combine at the positive electrode and deposit there as Li.
Li1 – x CoO2 + CLix →
LiCoO2 + C
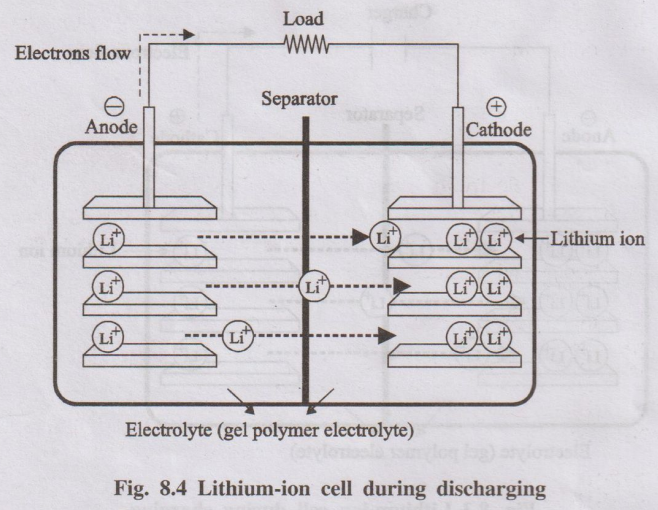
Fig. 8.4 Lithium-ion cell during discharging
Advantages (or) Characteristics of Lithium cell
1. Lithium-ion batteries are high voltage and light weight batteries.
2. It is smaller in size.
3. It produces three time the voltage of Ni-Cd batteries.
4. It has none of the memory effect seen in Ni-Cd batteries.
Uses of Lithium cell
It is used in cell phone, note PC, portable LCD TV, semiconductor
driven audio, etc.,
Engineering Chemistry: Unit V: b. Energy Storage Devices : Tag: Engineering Chemistry : Description, Diagram, Construction, Working Principle, Cell reactions, Advantages, Disadvantages, Uses - Important Secondary Batteries: Lead - Acid Storage Cell, Lithium-ion battery (LIB) (or) Lithium-ion cell
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
