Electromagnetic Theory: Unit III: (b) Magnetic Forces, Magnetic Materials and Inductance
Nature of Magnetic Materials
• In this section, we shall study the classification of the magnetic materials. Before actually starting with the classification, let us take a review of quantum theory in brief.
Nature of Magnetic Materials
AU
; Dec.-05, 09, 14, 17, May-11,18
•
In this section, we shall study the classification of the magnetic materials.
Before actually starting with the classification, let us take a review of
quantum theory in brief.
1. Origin of Magnetic Dipole Moment in the Material
•
Basically the magnetic materials are classified on the basis of presence of
magnetic dipole moments in the materials. A charged partical with angular
momentum always contributes to the permanent magnetic dipole moments. In
general, there are three important contributions to the angular moment of an
atom.
i)
Orbital magnetic dipole moment
ii)
Electron spin magnetic moment and
iii)
Nuclear spin magnetic moment
•
In any atom, several electrons revolve in the circular orbits around the
nucleus. This is very much analogous to a small current loop. The orbital state
of motion of an electron in an atom is described with the quantum numbers n, I
and ml. The first quantum number n indicates principle quantum which determines
the energy of an electron. The second quantum number I represents orbital
quantum number. Obviously it determines angular momentum of orbit called orbital angular momentum. The last quantum
number mZ is magnetic quantum number which determines the component of magnetic
moment along the direction of an external field. So a simple current loop,
placed in a magnetic field external to it, experiences a torque. Now this
torque tries to align the magnetic field produced by the orbital electrons with
an external field. Thus in general the orbital electrons produce magnetic field
in such a way to align with an external magnetic field with no other magnetic
moments considered.
•
The angular momentum of an electron is called spin of the electron. As electron
is a charged particle, the spin of the electron produces magnetic dipole
moment. In an atom with completely filled orbits the contribution in spin
magnetic moment is zero. In other words, the spins of the electrons in incompletely
filled shells contribute more in the resultant spin magnetic moment.
•
Similar to the electro spin, the nuclear spin contributes to the magnetic
moment called nuclear spin magnetic moment. The mass of the nucleus is much
larger than an electron. Thus the dipole moments due to the nuclear spin are
very small.
The
contribution of nuclear magnetic moment to the magnetic properties of materials
is negligible.
•
The total magnetic dipole moment of an atom can be calculated by summing up all
the above mentioned magnetic dipole moments in appropriate manner.
2. Classification of Magnetic Materials
•
According to the previous discussion, it is clear that the characteristics of
the magnetic materials are decided by the different components of moments and
also their summations. On the basis of the magnetic behaviour, the magnentic
materials are classified as diamagnetic, paramagnetic, ferromagnetic,
antiferromagnetic, ferrimagnetic and supermagnentic.
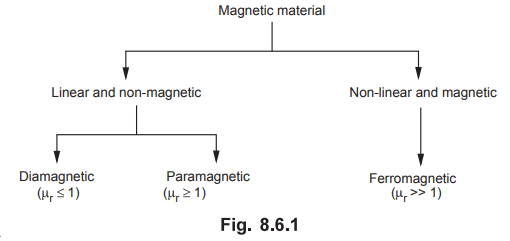
•
The magnetic materials can also be classified on the basis of magnetic property
namely relative permeability µr. In general, a material is said to
be non-magnetic if the value of µr is less than 1 or approximately
equal to 1. While a material is said to be magnetic if the value of µr
is greater than or equal to 1. Generally
the magnetic materials are classified into three major heads namely
diamagnetic, paramagnetic and ferromagnetic. If a value of µr is
slightly less than unity then it is a diamagnetic material (µr < l).
If the value of µr is slightly greater than unity, then it is
paramagnetic material (µr > 1). If the value of µr is
very large than unity (µr >> l), then it is ferromagnetic
marterial. For most of the practical cases, the value of µr is
assumed to be unity for a paramagnetic and diamagnetic materials. Hence
generally these materials are considered to be linear and non-magnetic
materials. On the other hand, the ferromagnetic materials are always non-linear
and magnetic materials. The classification of a magnetic material on the basis
of relative permeability of a material is as shown in Fig. 8.6.1.
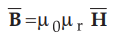
•
The magnetic materials in which the orbital magnetic moment and electron spin
magnetic moment cancel each other making net permanent magnetic moment of each
atom zero are called diamagnetic materials. Thus with no external field, in
diamagnetic materials the net torque produced on atom is zero with no effective
realignment of magnetic moment. But an applied field makes spin moment slightly
greater than that of orbital moment. This results in small magnetic moment
which opposes the applied field. Hence when a diamagnetic material like bismuth
is kept near either pole of a strong magnet gets repelled. Other examples of
diamagnetic materials are lead, copper, silicon, diamond, graphite, sulphur,
sodium chloride and inert gases.
•
The magnetic materials in which the orbital and spin magnetic moments do not
cancel each other resulting in a net magnetic moment of an atom are called
paramagnetic materials. In the paramagnetic materials, atoms are oriented
randomly. In the absence of an external field, the paramagnetic materials do
not show any magnetic effect. But when an external field is applied, each
atomic dipole moment experiences a torque. Due to this, all the atomic dipole moments
tend to align with the external field. Thus inside material, value of the field
increases than the value of the external field if the perfect alignment of the
dipole moments is achieved. When the paramagnetic material is kept near the
pole of a strong magnets, it gets attracted. The common examples of
paramagnetic materials are potassium, tungston, oxygen, rare earth metals.
•
The materials in which the atoms have large dipole moment due to electron spin
magnetic moments are called ferromagnetic materials. In the ferromagnetic
materials, the adjacent atoms line up their magnetic dipole moments in parallel
fashion in the lattice. The regions in which large number of magnetic moments
lined in parallel are called domains. When an external field is applied, the
domains increase their size increasing internal field to a high value. When the
external field is removed, the original random alignment of dipole moments is
not achieved. Some of the moments remain in a small region which results in
residual field or remanant field. This effect is called hysteresis. Iron,
nickel and cobalt are the examples of ferromagnetic materials.
•
By comparing ferromagnetic materials with diamagnetic and paramagnetic
materials, the ferromagnetic materials show following unique properties.
1)
These materials can be strongly magnetized with a magnetic field.
2)
Evenafter removing the magnetic field, these materials are capable of retaining
a considerable amount of magnetization.
3)
When these materials are heated above certain temperature, their ferromagnetic
properties are lost and these materials turn to be linear paramagnetic in
nature. Such a temperature is commonly called Curie temperature (For iron Curie
temperature is 770 °C)
4)
As these are non-linear materials, the relation ![]() fails in such
materials because the relative permeability µr depends on
fails in such
materials because the relative permeability µr depends on ![]() .
.
•
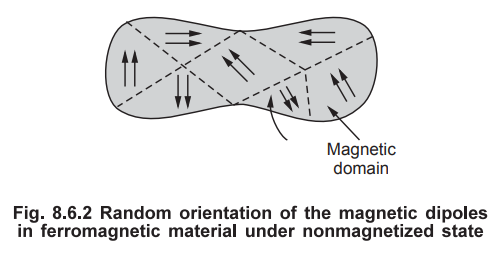
The behaviour of a ferromagnetic material can be represented interms of
magnetic domains. A magnetic domain is a small region in which all the magnetic
dipoles are perfectly aligned as shown in the Fig. 8.6.2. But the direction of
alignment of the dipoles vary from domain to domain. Hence this virgin material
is said to be in non-magnetized state.
•
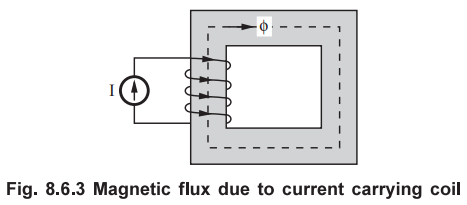
When a current carrying wire is wound around the magnetic material, a magnetic
field is produced. So when the magnetic material is placed in an external
magnetic field, all the magnetic dipoles will try to align in the direction of
magnetic field. The domains in the material, which are already in the direction
of magnetic field, grow in size at the cost of neighbouring domains. The
remaining domains rotate their dipoles in the direction of magnetic field. Thus
magnetic flux density within the magnetic material increases.
•
Due to the current in wire, ![]() is produced in material. The applied
field
is produced in material. The applied
field ![]() produces
produces ![]() field within the medium. The movement of the
domain walls is reversible till the
field within the medium. The movement of the
domain walls is reversible till the ![]() field within medium is weak.
field within medium is weak.
•
When the current in wire is increased, the ![]() field increases and
field increases and ![]() field becomes stronger and stronger. This is due to more number of magnetic
dipoles align with the
field becomes stronger and stronger. This is due to more number of magnetic
dipoles align with the ![]() field. Now if we measure
field. Now if we measure ![]() field, it
is observed that initially
field, it
is observed that initially ![]() field increases slowly, then it increases
rapidly. Then again slowly
field increases slowly, then it increases
rapidly. Then again slowly ![]() field increases and then it flattens off
finally as shown in the Fig. 8.6.4 (a).
field increases and then it flattens off
finally as shown in the Fig. 8.6.4 (a).

•
The changes in ![]() are due to the changes in
are due to the changes in ![]() is magnetization.
The flattened region indicate that almost all the magnetic dipoles are aligned themselves
in the direction of
is magnetization.
The flattened region indicate that almost all the magnetic dipoles are aligned themselves
in the direction of ![]() field.
field.
•
Now if we lower the![]() field by decreasing current in wire, the
field by decreasing current in wire, the ![]() field does not follow the same path but it slowly decreases as shown
by the dashed path in the Fig. 8.6.4 (a). Thus it is clear that even though the
field does not follow the same path but it slowly decreases as shown
by the dashed path in the Fig. 8.6.4 (a). Thus it is clear that even though the ![]() field becomes zero, there exists certain magnetic field density in material.
So it is called residual flux density or remanant flux density. It is denoted
by
field becomes zero, there exists certain magnetic field density in material.
So it is called residual flux density or remanant flux density. It is denoted
by ![]() . The magnetic material which retains high residual flux density is
called hard magnetic material.
. The magnetic material which retains high residual flux density is
called hard magnetic material.
•
When the direction of the current through wire is reversed, the flux density ![]() becomes zero at a certain value of
becomes zero at a certain value of ![]() in opposite direction.
The value of
in opposite direction.
The value of ![]() to make
to make ![]() zero is called coercive force denoted
by
zero is called coercive force denoted
by![]() . By increasing and decreasing
. By increasing and decreasing ![]() field in both the
directions, we get a loop which is called hysteresis loop. The area of this hysteresis
loop determines the loss in the energy per cycle which is known as hysteresis
loop. Thus to minimise the hysteresis loss, the area of the hysteresis loop
should be small. That means the residual flux density of the material should be
as small as possible. The material with small values of the residual flux
density is called soft material.
field in both the
directions, we get a loop which is called hysteresis loop. The area of this hysteresis
loop determines the loss in the energy per cycle which is known as hysteresis
loop. Thus to minimise the hysteresis loss, the area of the hysteresis loop
should be small. That means the residual flux density of the material should be
as small as possible. The material with small values of the residual flux
density is called soft material.
•
The materials in which the dipole moments of adjacent atoms line up in
antiparallel fashion are called antiferromagnetic materials. The net magnetic
moment in such materials is zero. Thus when a specimen of antiferromagnetic
material is kept near a strong magnet gets neither attracted nor repelled. This
property is observed in materials like many of the oxides, chlorides and
sulphides at low temperatures.
•
The materials in which the magnetic dipole moments are lined up in antiparallel
fashion, but the net magnetic moment is non-zero are called ferrimagnetic
materials. The specimen of ferrimagnetic material gets affected in the external
strong fields much lower than ferromagnetic materials. Ferrites are special
ferrimagnetic materials having very low electrical conductivity. So these
materials are used as ac inductors and core of transformers as the eddy
currents are reduced and ohmic losses are reduced. The common examples of
ferrites are nickel ferrite, nickel-zinc-ferrite and iron-oxide-magnetite.
•
In supermagnetic materials, the ferromagnetic materials are suspended in the
dielectric matrix. The important property of the supermagnetic material is that
eventhough each particle of it contain large magnetic domains but can not
penetrate adjacent particles. The common examples of supermagnetic materials
are magnetic tapes used for audio, video and data recordings.
Review Questions
1. Classify different magnetic materials with suitable examples.
2. Give a brief note on the magnetic materials.
AU : Dec.-09, 17, Marks 6
3. Discuss the phenomenon of hysteresis associated with
ferromagnetic materials.
AU : Dec.-05, Marks 8
5. Describe the
classification of magnetic materials and draw a typical magnetization (B-H)
curve.
AU : Dec.-14, Marks 8
Electromagnetic Theory: Unit III: (b) Magnetic Forces, Magnetic Materials and Inductance : Tag: : - Nature of Magnetic Materials
Related Topics
Related Subjects
Electromagnetic Theory
EE3301 3rd Semester EEE Dept | 2021 Regulation | 3rd Semester EEE Dept 2021 Regulation
