Engineering Chemistry: Unit III: a. Phase Rule
One Component System
water system | Phase Rule
Water exists in three possible phases namely solid, liquid and vapour. Hence, there can be three forms of equilibria.
ONE COMPONENT SYSTEM
1. The water system
Water exists in three possible phases namely solid, liquid and
vapour. Hence, there can be three forms of equilibria.
Solid ⇌ Liquid
Liquid ⇌ Vapour
Solid ⇌ Vapour
Each of the above equilibrium involves two phases. The phase
diagram for the water system is shown in the Fig. 3.1.
This phase diagram contains curves, areas, and triple point.
1. Curve OA
The curve OA is called vapourisation curve, it represents
the equilibrium between water and vapour. At any point on the curve the
following equilibrium will exist.
Water ⇌ Water vapour
The degree of freedom of the system univariant. This is predicted
by the phase rule.
F = C- P + 2; F = 1 - 2 +
2; F = 1
This equilibrium (i.e. line OA) will extend upto the critical
temperature (374°C). Beyond the critical temperature the equilibrium will
disappear only water vapour will exist.
2. Curve OB
The curve OB is called sublimation curve of ice, it
represents the equilibrium between ice and vapour. At any point on the curve
the following equilibrium will exist.
Ice ⇌ Vapour
The degree of freedom of the system is one, i.e. univariant. This
is predicted by the phase rule.
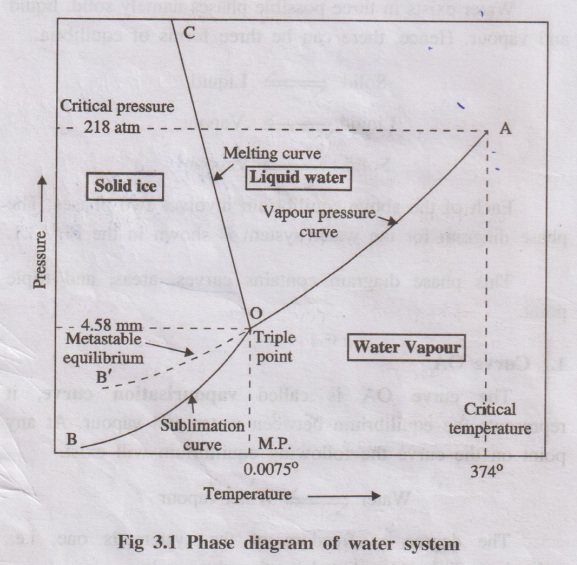
Fig 3.1 Phase diagram of water system
F = C - P + 2; F = 1 – 2 + 2; F = 1
This equilibrium (line OB) will extend upto the absolute zero (-
273°C), where no vapour can be present and only ice will exist.
3. Curve OC
The curve OC is called melting point curve of ice, it
represents the equilibrium between ice and water. At any point on the curve the
following equilibrium will exist.
Ice ⇌ water
The curve OC is slightly inclined towards pressure axis. This,
shows that melting point of ice decreases with increase of pressure.
The degree of freedom of the system is one, i.e., univariant.
4. Point ‘O' (Triple point)
The three curves OA, OB and OC meet at a point ‘O’, where three
phases namely solid, liquid and vapour are simultaneously at equilibrium.
This point is called triple point, at this point the
following equilibrium will exist.
Ice(s) ⇌ Water(1) ⇌ Vapour(g)
The degree of freedom of the system is zero i.e., nonvariant. This
is predicted by the phase rule.
F = C – P + 2; F = 1 – 3 + 2; F = 0
Temperature and pressure at the point “O’ are 0.0075°C and 4.58 mm
respectively:
5. Curve OB': (Metastable equilibrium)
The curve OB' is called vapour pressure curve of the super-cool
water or metastable equilibrium where the following equilibrium will exist.
Super - cool water ⇌ Vapour
Sometimes water can be cooled below 0°C without the formation of
ice, this water is called super-cooled water. Super cooled water is unstable
and it can be converted into solid by "seeding” or by slight disturbance.
6. Areas
Area AOC, BOC, AOB represents water, ice and vapour respectively.
In order to define the system at any point in the areas, it is essential to
specify both temperature and pressure. The degree of freedom of the system is
two. i.e., Bivariant. This is predicted by the phase rule
F = C – P + 2; F = 1 - 1 + 2; F = 2
Engineering Chemistry: Unit III: a. Phase Rule : Tag: Engineering Chemistry : water system | Phase Rule - One Component System
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
