Engineering Chemistry: Unit III: a. Phase Rule
Phase Rule
Definition of terms with examples
If the equilibrium between any number of phases is not influenced by gravity, or electrical, or magnetic forces but is influenced only by pressure, temperature and concentration, then the number of degree of freedom (F) of the system is related to number of components (C) and number of phases (P) by the following phase rule equation.
PHASE RULE
If the equilibrium between any number of phases is not influenced
by gravity, or electrical, or magnetic forces but is influenced only by
pressure, temperature and concentration, then the number of degree of freedom
(F) of the system is related to number of components (C) and number of phases
(P) by the following phase rule equation.
F = C – P + 2
1. Definition of terms with examples
1. Phase (P)
Phase is defined as, “any homogeneous physically distinct and
mechanically separable portion of a system which is separated from other parts
of the system by definite boundaries”.
Examples
(a) Gaseous phase
All gases are completely miscible and there is no boundary between
one gas and the other.
Example
Air, which is a mixture of O2, H2, N2,
CO2 and water vapour, etc., constitutes a single phase.
(b) Liquid phase
The number of liquid phases depends on the number of liquids
present and their miscibilities.
(i) If two liquids are immiscible, they will form three separate
phases two liquid phase and one vapour phase.
Example
Benzene - Water.
(ii) If two liquids are completely miscible, they will form one
liquid phase and one vapour phase.
Example
Alcohol - Water.
(c) Solid phase
Every solid constitutes a separate phase.
Example
Decomposition of CaCO3
CaCO3(s) ⇌ CaO(s) + CO2(g)
It involves three phases, solid CaCO3, solid CaO and
gaseous CO2.
(d) Consider a water system consisting of three phases.
Ice(s) ⇌ Water(1) ⇌ Vapour(g)
Each phase is physically distinct and homogeneous and there are
definite boundaries between phases. So this forms three phases.
(e) A solution of a substance in a solvent consists of one phase
only.
Example
Sugar solution in water.
(f) An emulsion of oil in water forms two phases
(g) MgCO3 (8) → MgO(s) + CO2(g)
It involves three phases, solid MgCO3, solid MgO and
gaseous CO2
(h) Rhombic sulphur (s) → Monoclinic sulphur (s)
It forms two phases.
(i) Consider the following heterogeneous system.
CuSO4(s) + 5H2O(1)
⇌ CuSO4 . 5H2O(s)
Number of phase = 3; Number of component = 2
2. Component (C)
Component is defined as, “the smallest number of independently
variable constituents, by means of which the composition of each phase can be
expressed in the form of a chemical equation”.
Examples
(a) Consider a water system consisting of three phases.
Ice(s) ⇌ Water(1) ⇌ Vapour(g)
The chemical composition of all the three phases is H2O,
but are in different physical form. Hence the number of component is one.
(b) Sulphur exists in 4 phases namely rhombic, monoclinic, liquid
and vapour, but the chemical composition is only sulphur. Hence it is a one
component system.
(c) Thermal decomposition of CaCO3
CaCO3(s) ⇌ CaO(s) + CO2(g)
The system consists of three phases namely, solid CaCO3,
solid CaO and gaseous CO2. But it is a two component system, because
the composition of each of the above phases can be expressed in terms of any
two of the three components present. When CaCO3 and CaO are
considered as components.
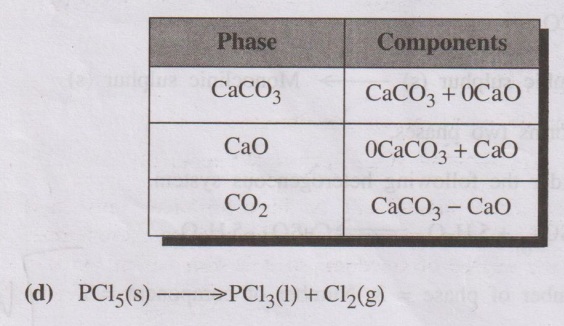
This system has three phases, but the number of component is only
two.
(e) An aqueous solution of NaCl is a two component system.
The constituents are NaCl and H2O.
(f) CuSO4.5H2O(s) ⇌ CuSO4 . 3H2O(s) + 2H2O(g)
It is also a two component system.
(g) In the dissociation of NH4C1, the following
equilibrium occurs.
NH4Cl(S) ⇌ NH3(g) + HCl(g)
The system consists of two phases namely solid NH4Cl and the
gaseous mixture containing NH3 + HCl. When NH3 and HCl
are present in equivalent quantities the composition of both the phases can be
represented by the same chemical compound NH4Cl and hence the system
will be a one component system.
3. Degree of freedom (F)
Degree of freedom is defined as, "the minimum number of
independent variable factors such as temperature, pressure and concentration,
which must be fixed in order to define the system completely”.
A system having 1, 2, 3 and 0 degrees of freedom is called
univariant, bivariant, trivariant and nonvariant respectively.
Examples
(a) Consider the following equilibrium
Ice(s) ⇌ Water(1) ⇌ Vapour(g)
These three phases will be in equilibrium only at a particular
temperature and pressure. Hence, this system does not have any degree of
freedom, so it is non variant or zero variant.
(b) Consider the following equilibrium
Water(1) ⇌ Water vapour(g)
Here liquid water is in equilibrium with water vapour. Hence any
one of the degrees of freedom such as temperature or pressure has to be fixed
to define the system. Therefore the degree of freedom is one.
(c) For a gaseous mixture of N2, and H2, we
must state both the pressure and temperature. Hence, the system is bivariant.
Engineering Chemistry: Unit III: a. Phase Rule : Tag: Engineering Chemistry : Definition of terms with examples - Phase Rule
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
