Engineering Chemistry: Unit V: a. Energy Sources
Solar Cell or Photogalvanic Cell
Definition, Working Principle, Construction, Applications, Advantages, Disadvantages, Merits, Demerits
Solar cell (or) Photogalvanic cell is the one, which converts the solar energy (energy obtained from the sun) directly into electrical energy.
SOLAR CELL (or) PHOTOGALVANIC CELL
Definition
Solar cell (or) Photogalvanic cell is the one, which converts the
solar energy (energy obtained from the sun) directly into electrical energy.
Principle
The basic principle involved in the solar cells is based on the
photovoltaic (PV) effect. When the solar rays fall on a two layer of
semi-conductor devices, a potential difference between the two layer is
produced. This potential difference causes flow of electrons and produces
electricity.
Construction
Solar cells consist of a p-type semiconductor (such as Si doped
with B) and n-type semiconductor (such as Si doped with P). They are in close
contact with each other.
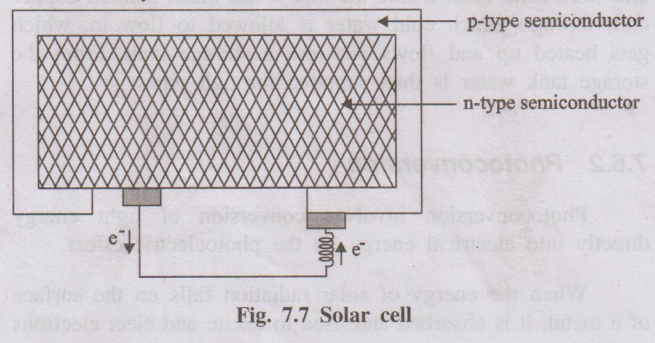
Fig. 7.7 Solar cell
Working
When the solar rays fall on the top layer of p-type semiconductor,
the electrons from the valence band get promoted to the conduction band and
cross the p-n junction into n-type semiconductor. There by potential difference
between two layers is created, which causes flow of electrons (ie., an electric
current). Thus, when the p and n layers are connected to an external circuit,
electrons flow from n-layer to p-layer, and hence current is generated.
The potential difference and hence current increases as more solar
rays falls on the surface of the top layer.
1. Applications of solar cells
1.
Lighting purpose
Solar cells can be used for lighting purpose. Now a days
electrical street lights are replaced by solar street lights.
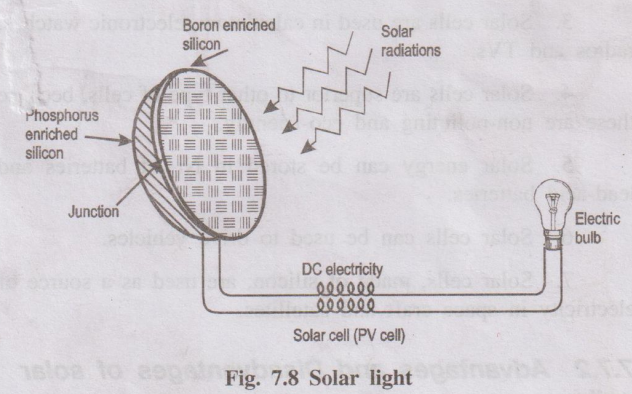
Fig. 7.8 Solar light
2. Solar pumps run by solar battery
When a large number of solar cells are connected in series it form
a solar battery. Solar battery produces more electricity which is enough to
run, water pump, street-light,
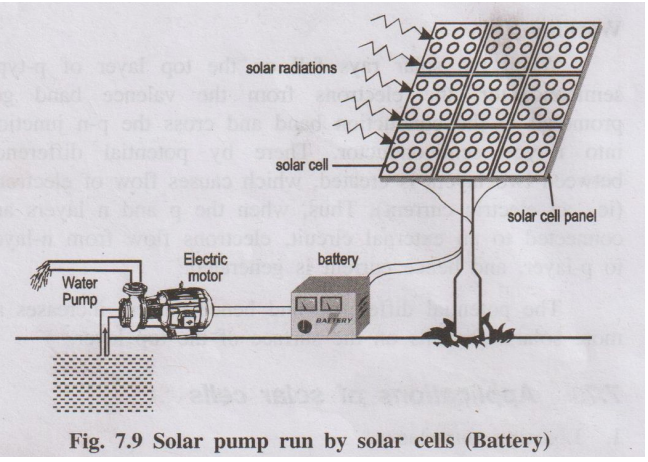
Fig. 7.9 Solar pump run by solar cells (Battery)
etc., They are also used in remote areas where conventional
electricity supply is a problem.
3. Solar cells are used in calculators, electronic watches, radios
and TVs.
4. Solar cells are superior to other type of cells, because these
are non-polluting and eco-friendly.
5. Solar energy can be stored in Ni-Cd batteries and lead-acid
batteries.
6. Solar cells can be used to drive vehicles.
7. Solar cells, made of silicon, are used as a source of
electricity in space craft and satellites.
2. Advantages and Disadvantages of solar cells
Advantages (or) Merits
1. Solar cells can be used in remote and isolated areas, forests
and hilly regions.
2. Maintenance cost is low.
3. Solar cells are noise and pollution free.
4. Their lifetime is long.
5. They operate at ambient temperature.
6. They need not be recharged.
Disadvantages (or) Demerits
1. Capital cost is higher.
2. Storage of solar energy is not possible.
3. It produces only DC voltage.
4. Solar energy is not available throughout day and night.
Engineering Chemistry: Unit V: a. Energy Sources : Tag: Engineering Chemistry : Definition, Working Principle, Construction, Applications, Advantages, Disadvantages, Merits, Demerits - Solar Cell or Photogalvanic Cell
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
