Engineering Chemistry: Unit V: a. Energy Sources
Stability of Nucleus: Mass defect, Binding energy
Definition, Calculation, Solved Example Problems
It has been found that the actual mass of an isotope is always less than the sum of the masses of the protons, neutrons and electron from which it is formed.
Unit – V
Chapter 7
Energy Sources
INTROUDUCTION
For our energy requirement, we mainly depond on the conventional
sources of energy like coal, petroleum, natural gas, etc., These sources are
limited in quantity and can be exhausted in near future. This is because of
their continuous and rapid use.
To overcome this energy crises the scientists have accelerated the
search and use of non- conventional (renewable) sources of energy, solar
energy, nuclear energy, etc,
STABILITY OF NUCLEUS
1. Mass defect
It has been found that the actual mass of an isotope is always
less than the sum of the masses of the protons, neutrons and electron from
which it is formed.
Definition
The difference between the calculated and experimental masses of
nucleus is called mass defect. It is denoted by ∆m.
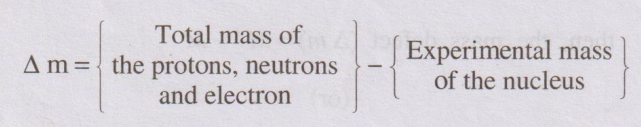
(or)
It is defined as the loss of mass during the formation of the
nucleus of the isotope.
Calculation of mass defect
Consider an isotope,
let its atomic number = Z
Mass number = A
If its atom contains
Z protons, Z electrons and (A-Z) neutrons
Let,
mp = mass of proton
mn = mass of neutron
me = mass of an electron
ஃ Calculated
mass of isotope
M' = Z mp + Zme + (A - Z)mn
= ZmH + (A - Z) mn
(where, mp + me = mass of H atom = mH)
Let,
M = Actual experimental mass of the nucleus,
then, the mass defect (A m) = M'-M
(or)
∆m= ZmH + (A - Z) mn-M
Problems on Mass defect
1. Calculate the mass defect of 20Ca40 ,
which has atomic mass of 39.975 amu. The mass of proton is 1.0078 amu and the
mass of neutron is 1.0086 amu.
Solution
Mass defect (∆m) of Ca-atom = Zmp + (A - Z) mn -
M
Note
Calcium atom is composed of 20 protons and 20 neutrons.
Given: Z = 20; (A - Z) = 20; mH
= 1.0078 amu ;
mn = 1.0086 amu; M = 39.975 amu;
ஃ ∆m = [ 20 mp + 20 mn -M] [me =
Neglected]
= [20 × 1.0078 + 20 × 1.0086 – 39.975]
= [20.156 + 20.172 – 39.975]
= 40.328 – 39.975
= 0.353 amu
2. Calculate the mass defect of 2He4, if its
experimentally determined mass is 4.00390 amu. The masses of a proton, an
electron and a neutron are 1.007825, 0.0005852 and1.008668 amu respectively.
Solution
Mass defect (Am) of He-atom = [2m, + 2me + 2mn-M]
Note
2He4 atom is
composed of 2 protons, 2 electrons and 2 neutrons.
Given: mp = 1.007825
amu; me = 0.0005852 amu ;
mn = 1.008668 amu
ஃ ∆m= [2 ×
1.007825 + 2 × 0.0005852 + 2 × 1.008688 - 4.00390] amu
= [4.0341964 – 4.00390] amu
= 0.0302964 amu
3. Mass of hydrogen and neutron are 1.008 amu and 1.0072 amu
respectively. If experimentally determined mass of sodium atom (11Na23)
is 23.0092 amu. Calculate mass defect.
Solution
Mass defect of Na-atom
(∆m) = [ 11 × mH + 12mn – M ]
Note
11Na23 is made up
of 11-H-atom and 12-neutrons.
Given: mp (or) mH
= 1.008 amu; mn = 1.0072 amu ;
M= 23.0092
ஃ ∆m = [ 11 × 1.008 + 12 × 1.0072 – 23.0092 ] amu
= 0.1652 amu
4. Calculate the mass defect of -particles. Given that the mass of
proton = 1.00758 amu, mass of neutron = 1.00897 amu and mass of helium nucleus =
4.00820 amu.
Solution
Mass defect (∆m) of ɑ -particle = [2mp + 2mn
- M]
Note
An ɑ - particle is composed of 2 protons and 2 neutrons
Given: mp = 1.00758
amu; mn= 1.00897 amu ;
M = 4.00820 amu
ஃ∆m = [ 2 ×
1.00758 + 2 × 1.00897 – 4.00820 ) amu
= 0.02490 amu
5. Calculate the mass defect of the following fission reaction.
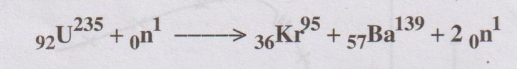
Given that atomic mass of U235 = 235.124 amu;
Kr95 = 94.945 amu ; Ba139 = 138.954 amu; 0n1
= 1.0099 amu
Solution
ஃ ∆m= Atomic
mass of [U+n - (Kr + Ba + 2n)]
= [235.124 + 1.0099 – (94.945 + 138.954 + 2 × 1.0099)]
= 236.1339 – (233.899 + 2.0198)
= 236.1339 - 235.9188
= 0.2151 amu
2. Binding energy
Loss of mass during the formation of nucleus from nucleons gets
converted into energy. This energy is known as binding energy. Binding energy
is responsible for the stability of the nucleus. The greater the energy liberated
the greater is the stability of the nucleus. Hence, binding energy (BE) is the
energy equivalent of mass defect.
Definition
Binding energy is defined as the energy released when a given
number of protons and neutrons coalesee to form nucleus.
(or)
It is the energy required to disrupt the nucleus into its
constituent protons and neutrons.
Binding Energy Vs Nuclear Stability
A plot of Binding energy per nucleon against mass number for
different elements gives the following graph:
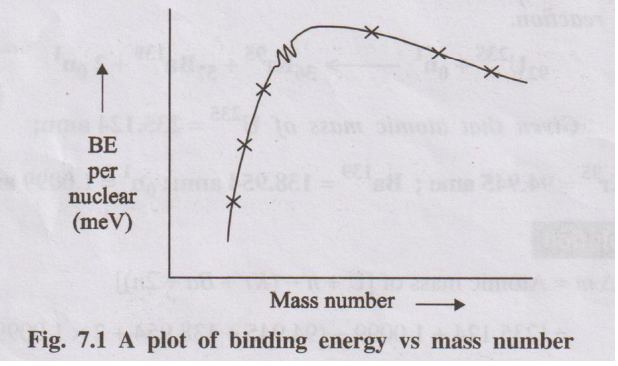
Fig. 7.1 A plot of binding energy vs mass number
The graph shows that BE and hence the stability of nucleus
increases upto a mass number of 65 and decreases thereafter. There are some
subsidiary peaks in the plot at 2He4, 6C12
and 8O16 indicating stable nuclear configurations. This
is probably due to the presence of equal number of protons and neutrons.
Calculation of binding energy
Binding energy can be calculated from the mass defect using the
relation.
E = ∆ m c2
E = [ZmH + (A - Z) mn - M] × c2
where, E - Binding energy of the nucleus
∆ m - Mass defect
c - Velocity of light (3 × 108 m/s)
Engineering Chemistry: Unit V: a. Energy Sources : Tag: Engineering Chemistry : Definition, Calculation, Solved Example Problems - Stability of Nucleus: Mass defect, Binding energy
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
