Engineering Chemistry: Unit II: Nanochemistry
Types of Nano Materials
with their Examples, Structures, Applications, Uses
Based on dimensions, nano materials are classified in to four types.
TYPES OF NANO
MATERIALS
Based on dimensions, nano materials are classified in to four
types.
1. Nanoparticles
2. Nanoclusters
3. Nanowire
4. Nanorods
5. Nanotubes
Co-ordinates : Examples
1. 0 – dimension : Nanoclusters
2. 1 - dimension : Thin Films, surface coatings
3. 2 - dimension : Nanotubes, nanowires
4. 3 - dimension : Precipitates,
colloids
1. Nanoparticles
Nanoparticles are the particles, the size of which ranges from 1
to 100 nm. These are tiny aggregates of atoms but smaller than their crystals
but bigger than molecules. They have three dimensional structures.
Examples : TiO2, gold,
silver, ZnO, etc.,
Applications of
Nanoparticles
1. TiO2 is used in cosmetics
as they are very good UV - absorber.
2. Nano silver particles are used as a catalyst in industries.
3. Nanoparticles are used in medicine.
Nano silver particles are used in making bone cement, surgical
instruments, etc.,
2. Nanoclusters
Nanoclusters are fine aggregates of atoms or molecules. The size
of which ranges from 0.1 to 10 nm. Of all the nano materials, nanoclusters are
the smallest sized nano materials because of their close packing arrangement of
atoms.
Examples : CdS, ZnO, etc.,
All the atoms, in nanocluster, are bound by forces like metallic,
covalent, ionic, hydrogen bond or Vander Waals forces of attraction. Clusters
of certain critical size are more stable than others. Nanoclusters consisting
of up to a couple of hundred atoms, but larger aggregates, containing 10° or
more atoms, are called nanoparticles.
Magic number
Magic number is the
number of atoms present in the clusters of criticle sizes with higher
stability.
Different types of nanoclusters can be distinguished from the
nature of forces present between atoms. Generally clusters containing
transition metal atom have unique chemical, electronic and magnetic properties.
These properties vary with the number of constituent atoms, the type of element
and the net charge on the cluster.
Properties of
nanoclusters
1. Atomic clusters or molecular clusters
are formed by the nucleation of atoms or
molecules respectively.
2. The reactivity of nanoclusters are decreased due to their decrease in size.
3. The melting
point of nanoclusters are lower than the bulk materials due to high surface to
volume ratio.
4. The electronic structure of the nanocluster is more confined
than the bulk materials.
Applications of nanocluster
1. Nanoclusters are used as catalysts in many reactions.
2. It is used in nano based chemical sensors.
3. It is also used as a light emitting diode in quantum computers.
3. Nanorods
Nanorod is two dimensional cylindrical solid material having an
aspect ratio i.e., length to width ratio less than 20.
Examples : Zinc oxide, Cadmium
sulphide, Gallium nitride nano rods.
Synthesis of nanorods
Nano-rods are produced by direct chemical synthesis. A combination
of ligands act as shape control agents and bond to different facets of the
nano-rods with different strength.
This allows different nanorods to grow at different rates
producing an elongated objects. Many of the above nanorods are not manufactured
due to lack of commercial demand.
Properties of nanorods
1. Nanorods are two-dimensional materials.
2. It exhibits optical and electrical properties.
Applications of
nanorods
1. Nanorods find application in display
technologies.
2. It is also used in the manufacturing of micro mechanical switches.
3. Nanorods are used in an applied electric field, micro electro
mechanical systems, etc.,
4. Nanorods along with noble metal nanoparticles function as
theragnostic agents.
5. They are used in energy harvesting and light emitting devices.
6. Nanorods have used as cancer therapeutics.
4. Nanowires
Nanowire is two dimensional cylindrical solid material having an
aspect ratio ie., length to width ratio greater than 20. Diameter of the
nanowire ranges from 10 - 100 nm.
Examples : Different types of nanowires
Types of nanowires : Examples
1. Metallic nanowires : Au, Ni, Pt
2. Nanowires of semiconductors : InP, Si, GaN
3. Nanowires of insulators : SiO2, TiO2
4. Molecular nanowires : DNA
Synthesis of nanowires
1. Template-assisted
synthesis
Template assisted synthesis of nanowires is a simple way to
fabricate nanostructures. These templates contain very small cylindrical pores
or voids within the host material and the empty spaces are filled with the
chosen material to form nanowires.
2. VLS (Vapour - Liquid
- Solid) method
It involves the absorption of the source material from the gas
phase into a liquid phase of catalyst. Upon supersaturation of the liquid
alloy, a nucleation event generates a solid precipitate of the source material.
This seed serves as a preferred site for further deposition of material at the
interface of the liquid droplet, promoting the elongation of the seed into a
nanowire.
Properties of nanowires
1. Nanowires are two-dimensional material.
2. Conductivity of a nanowire is less than that of the
corresponding bulk materials.
3. It exhibits distinct optical, chemical, thermal and electrical
properties due to this large surface area.
4. Silicon nanowires show strong photoluminescence
characteristics.
Uses of nanowires
1. Nanowires are used for enhancing mechanical properties of
composites.
2. It is also used to prepare active electronic components such as
p-n junction and logic gates.
3. Semiconductor nanowire crossings are expected to play a
important role in future of digital computing
4. Nanowires find applications in high-density data storage either
as magnetic read heads or as patterned storage media.
5. Nanowires replace conventional copper wires used in computers,
televisions.
6. It is also used to link tiny components into very small
circuits.
5. Nanotubes
Nanotubes are tube like structures with diameter of 1-100 nm and a
length of few nm to microns. Nanotubes consist of tiny cylinders of carbon and
other materials like boron nitride. Nanotubes may be organic (or) inorganic.
Examples
1. Carbon nanotube
2. Silicon nanotube
3. DNA nanotube
4. Boron nitride nanotube
1. Carbon nanotubes (CNTs)
Carbon nanotube is a tubular form of carbon with 1-3 nm diameter
and a length of few nm to microns. Generally carbon in the solid phase exits in
different allotropic forms like graphite, diamond, fullerence and nano tubes.
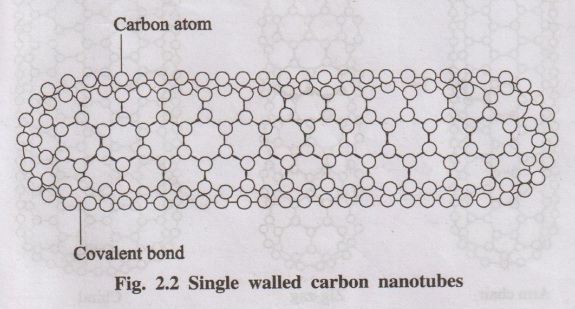
Fig. 2.2 Single walled
carbon nanotubes
Carbon nanotubes are tubular forms of carbon. When graphite sheets
are rolled into a cylinder, their edges join to each other form carbon
nanotubes. Each carbon atom in the carbon nanotubes is linked by covalent
bonds. But the number of nanotubes align into ropes and are held together by
weak Vander Walls forces.
Structures (or) types
of carbon nanotubes
Depending upon the way in which graphite sheets are rolled, two
types of CNTs are formed.
1. Single - walled nanotubes (SWNTS).
2. Multi - walled nanotubes (MWNTs).
1. Single - walled nanotubes (SWNTS)
SWNTs consist of one tube of graphite. It is one-atom thick having
a diameter of 2 nm and a length of 100 um. SWNTs are very important, because
they exhibit important electrical properties. It is an excellent conductor.
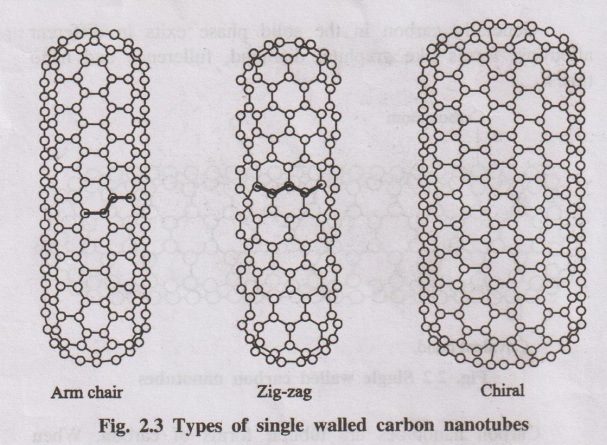
Fig. 2.3 Types of
single walled carbon nanotubes
Three kinds of nanotubes are resulted, based on the orientation of
the hexagon lattice.
(a) Arm-chair structures: The
lines of hexagons are parallel to the axis of the nanotube.
(b) Zig-zag structures: the
centre: The lines of carbon bonds
are down the center.
(c) Chiral nanotubes: It
exhibits twist or spiral around the nanotubes.
It has been confirmed that arm-chair carbon nanotubes are metallic
while zig-zag and chiral nanotubes are semiconducting
2. Multi - walled
nanotubes (MWNTs)
MWNTs (nested nanotubes) consist of multiple layers of graphite
rolled in on themselves to form a tube shape. It exhibits both metallic and
semiconducting properties. It is used for storing fuels such as hydrogen and
methane.

Fig. 2.4 Multiwalled
carbon nanotubes
Synthesis of Carbon
Nanotubes
Carbon nanotubes can be synthesized by the following methods.
1. Pyrolysis of hydrocarbons.
2. Laser evaporation.
1. Pyrolysis
Carbon nanotubes are synthesized by the pyrolysis of hydrocarbons
such as acetylene at about 700°C in the presence of Fe-silica or Fe-graphite
catalyst under inert conditions.
2. Laser evaporation
It involves vapourization of graphite target, containing small
amount of cobalt and nickel, by exposing it to an intense pulsed laser beam at
higher temperature (1200°C) in a quartz tube reactor. An inert gas such as
argon (or) helium is simultaneously allowed to pass into the reactor to sweep
the evaporated carbon atoms from the furnace to the colder copper collector, on
which they condense as carbon nanotubes.
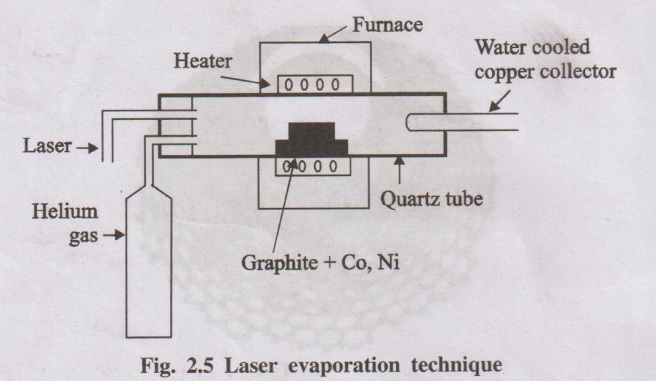
Fig. 2.5 Laser
evaporation technique
Properties of CNTS
1. CNTs are very strong, withstand extreme strain in tension and
posses elastic flexibility.
2. The atoms in a nano-tube are continuously vibrating back and
forth.
3. It is highly conducting and behaves like metallic or
semiconducting materials.
4. It has very high thermal conductivity and kinetic properties.
Uses of CNTs
1. It is used in battery technology and in industries as catalyst.
2. It is also used as light weight shielding materials for
protecting electronic equipments.
3. CNTs are used effectively inside the body for drug delivery.
4. It is used in composites, ICs.
5. It also acts as an efficient catalysts for some chemical
reactions.
6. It acts as a very good biosensor. Due to its chemical inertness
carbon nanotubes are used to detect many molecules present in the blood.
7. It is also used in water softening process as a filter.
Engineering Chemistry: Unit II: Nanochemistry : Tag: Engineering Chemistry : with their Examples, Structures, Applications, Uses - Types of Nano Materials
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
