Engineering Chemistry: Unit I: Water and its Treatment
Water Quality Parameters
The quality of water is a very important parameter to be determined in order to decide the type of application or treatment required. The quality of water varies to place to place and seasons.
WATER QUALITY PARAMETERS
The quality of water is a very important parameter to be
determined in order to decide the type of application or treatment required.
The quality of water varies to place to place and seasons.
The followings are some important parameters of quality of water.
1. Colour
Colour is a shade
imparted by organic or inorganic material, which change the appearance of the
water.
Colour is found mostly in surface water. The colours of natural
water range from pale straw through yellowish-brown to dark brown. The colour
of natural waters is mainly due to the presence of dissolved or colloidal
organic or inorganic materials.
Sources
1. Organic sources ⇒ Algae, tannins, humic compounds organic dyes, etc.
2. Inorganic sources ⇒ Fe and Mn compounds, chemicals and inorganic dyes from various
industries.
Sanitary Significance
1. The colours and the materials which produce colour are often
objectionable in which the water and the manufactured product come into
contact.
e.g., Dyeing, scouring and laundering
2. Variation in colour of water from the same source with time
serves as index of quality of the water.
e.g.,
(a) Yellowish tinge ⇒ indicates the presence of ‘Cr' and
organic matter.
(b) Yellowish red ⇒ indicates the presence of
iron.
(c) Red-brown ⇒ indicates the presence of peaty matter.
Removal of colour
Colour and colour producing materials are removed by coagulation,
settling, adsorption and filtration.
2. Tastes and Odours
Taste
Taste is the sensation
of flavour perceived in the mouth and throat on contact with a substance.
Odour
Odour is a smell (or)
scent caused by one (or) more volatilized chemical compounds that are generally
found in low concentration.
Sources
Organic sources: Algae
and decaying vegetation, etc.
Inorganic sources:
Mercaptans, amines and sulphides, etc.
The tastes and odours observed in chlorinated waters are due to
chloro-organic compounds formed by the reaction between chlorine and organic
matter present in the water.
How to Evaluate the Odour?
It is impossible to isolate and identify clearly the odour causing
materials. Evaluation of odour depends on the olfactory senses of the testing
person and on his ability to distinguish between different types of odours.
Significance
1. Disagreeable odours and tastes are objectionable for various
industrial processes such as food products, beverages, textiles, paper, pulp.
Most of the odours in natural waters are organic in nature, except H2S.
2. Tast helps us to decide what to eat and influences how efficiently
we digest these foods.
3. Senses of smell and taste are vital in identification of
valuable nutrients in the environment.
4. Taste enables the evaluation of foods for toxicity.
Removal of Tastes and Odours
(i) Organic tastes and odours may be removed by aeration (or)
activated carbon treatment.
(ii) Inorganic tastes due to H2S (or) Iron may be
removed by chemical methods like oxidation, chlorination (or) precipitation
3. Turbidity and sediments
Turbidity is the
reduction of clarity of natural water due to the presence of finely divided,
insoluble impurities suspended in water.
Sources
1. Inorganic sources
Clay, silt, silica, ferric hydroxide, calcium carbonate, sulphur,
etc.,
2. Organic sources
Finely divided vegetable or animal matter, oils, fats, greases,
micro-organisms, etc.,
Problems Caused by Turbidity
(i) Presence of turbidity and sediments in boiler water and cooling
water system cause problems.
(ii) Water-softening processes cannot be carried out.
(iii) Due to deposition of these organic impurities, disinfection
efficiency gets reduced.
Significance
(i) Turbidity affects the taste and odour of drinking water.
(ii) As turbidity affects the disinfection process, it must be removed.
(iii) Turbidity have many negative effects on aquatic life, it block
light to aquatic plants, aquatic organisms.
(iv) Turbidity affects the growth rate of algae.
(v) It increases water
temperature because suspended particles absorbs more heat.
Removal of Turbidity and Sediments
Turbidity of water may be removed by sedimentation followed by
(a) Coagulation and filtering
(b) Coagulation and settling
(c) Coagulation, settling and filtering.
Example
1. Turbidity caused by suspended silt and mud is objectionable in
boilers and in cooling-water systems.
2. Turbidity caused by colloidal or dissolved organic matter will
interfere with water-softening processes.
4. pH
The hydrogen ion
concentration is represented by the pH value, which is defined as
pH = - log10[H+]
pH is defined as negative logarithm of hydrogen ion concentration.
The pH value ranges from 0-14 as
0 < → 7 < →14
Acidic < -------- Neutral → Basic
Generally pH of natural waters lies in the neutral range. For
drinking water recommended pH = 6.5 to 8.5. For irrigation recommended pH = 6.0
to 9.0. Some surface waters passing over areas rich in sodium and potassium
posses alkaline pH. The rain water contaminated by the dissolved gases such as
SO2 and NOx will have acidic pH.
Significance of pH
(i) pH determines the solubility (amount that can be dissolved in
water).
(ii) It also determines the biological availability (amount that can
be utilized by aquatic life).
(iii) A rise (or) fall in pH can indicate chemical pollution (or)
acid rain. Many animals cannot live in water at a pH level below 5 (or) above
9.
5. Alkalinity
Alkalinity of water is
a measure of its acid-neutralising ability. The natural alkalinity in waters is
imparted by the hydroxides, carbonates and bicarbonates.
Sources
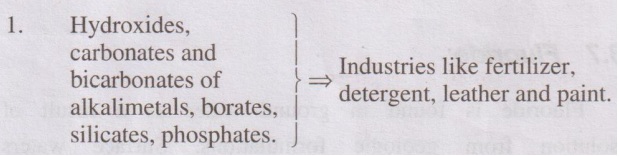
1. Hydroxides, carbonates and bicarbonates of alkalimetals, borates,
silicates, phosphates. } ⇒ Industries like fertilizer, detergent, leather and paint.
Sanitary significance
1. Very high values of alkalinity are harmful to aquatic
organisms.
2. Alkalinity in boiler feed water causes caustic embrittlement of
pipes.
Removal of Alkalinity
Alkalinity in water, can be removed by adding limited amount of
HC1.
6. TDS
TDS stands for Total
Dissolved Solids. It is defined as the measure of all inorganic and organic
substances present in water.
Significance
(i) TDS impacts the salinity of water.
(ii) TDS in water may not be ideal for your health and must be
filtered out before intake.
(iii) Water with TDS level higher than 300 ppm may not be potable
as it can taste salty.
(iv) Water with high level of TDS (> 1200) impacts the color,
odour and taste dramatically.
(v) Water with high level of TDS may not be suitable due to
excessive scaling caused by it in water pipes, heaters, boilers and household
appliances.
7. Fluoride
Fluoride is found in ground water as a result of dissolution from
geologic formulations. Surface waters generally contain much smaller
concentration of fluoride.
Sources
Fluoride containing minerals } ⇒ Fluorapatite (Ca10
F2 (PO4)6),
cryolite (Na3 AlF6) and igneous rocks
containing fluosilicates.
Contaminated domestic sewage, run-off from agricultural lands } ⇒ Phosphate fertilizers
Sanitary significance
1. Optimum fluoride concentrations, prescribed in public water
supplies, are in the range of 0.7 to 1.2 mg / lit.
2. Beneficial health effects have been observed where the fluoride
levels are optimum.
3. If the fluoride concentration is low in drinking water it causes
dental caries in children.
4. If the fluoride concentration is high it causes fluorosis.
Removal of fluoride (Defluoridation)
1. Precipitation using aluminium salts in alkaline media.
2. Using strongly basic anion exchange resin.
3. By adsorption on activated carbon.
8. Arsenic
Arsenic is a metallic element that forms a number of poisonous
compounds. It is found in nature at low levels, mostly in compounds, with
oxygen, chlorine and sulfur.
Source
Arsenic can get into drinking water from natural deposits (or)
runoff from agriculture, mining and industrial processes.
Significance
(i) Long-term intake of arsenic contaminated water leads to arsenic
poisoning with cancer of skin, bladder, kidney.
(ii) Association of arsenic contaminated water produces diabetes,
hypertension and reproductive disorders.
(iii) Children may have more exposure to arsenic in drinking water.
As a result, children may be at greater risk of illness when higher levels of
arsenic are present.
9. Chemical Oxygen Demand (COD)
COD is defined as, “the
measure of amount of oxyge required to chemically oxidise all the oxidisable
impuriti present in the sewage using an oxidising agent like acidifie K2Cr2O7”.
Significance of COD
(i) Determination of COD is carried out only in 3 hour but
determination of BOD is carried out after 5 days.
(ii) It measures both the biologically oxidisable and biologically
inert organic matter.
(iii) COD test is used to monitor water treatment plant efficiency.
(iv) COD is used to measure pollutants in water, waste water and
aqueous hazardous wastes.
(v) It provides an index to assess the effect of discharge waste
water on the environment.
10. Biological Oxygen Demand (BOD)
BOD is defined as, “the
amount of free oxygen require by bacteria for the biological oxidation of the
organic matte under aerobic conditions at 20°C for a period of 5 days”.
Significance of BOD
(i) It indicates the amount of decomposable organic matter present
in the sewage.
(ii) It enables us to determine the degree of pollution at any time
in the sewage stream.
(iii) Lesser the BOD, better is the quality of water. ie. the water
sample with BOD of less than 3 ppm is considere as pure water, whereas the
water more than 4 ppm i considered as polluted water.
11. Hardness of Water
Hardness is the
property (or) characteristics of water, which does not produce lather with
soap.
Types of hardness
Depending upon the types of dissolved salts present in water,
hardness of water can be classified into two types
1. Temporary hardness.
2. Permanent hardness.
1. Temporary hardness
(or) Carbonate hardness (CH) (or) Alkaline hardness
This is due to the presence of bicarbonates of calcium and
magnesium. It can be removed by (i) boiling the water (ii) adding lime to the
water.
2. Permanent hardness
(or) Non-carbonate hardness (NCH) (or) Non-alkaline hardness
This is due to the presence of chlorides and sulphates of calcium
and magnesium. It cannot be removed by boiling the water. But, it can be
removed by (i) Lime-soda process (ii) Zeolite process.
Significance of
Hardness
1. Hardness affects the amount of soap
that is needed to produce foam (or) lather.
2. Hardness is very
important in industrial uses, because it forms scale in heat exchange equipment
boilers and pipe lines.
3. Hardness mitigates metal toxicity because Ca2+ and
Mg2+ help keep fish from absorbing metals such as lead, arsenic and
cadmium into their blood stream.
Engineering Chemistry: Unit I: Water and its Treatment : Tag: Engineering Chemistry : - Water Quality Parameters
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
