Engineering Chemistry: Unit IV: a. Fuels
Analysis of Coal
In order to assess the quality of coal the following two types of analysis are made.
ANALYSIS OF COAL
In order to assess the quality of coal the following two types of
analysis are made.
1. Proximate Analysis
It is the analysis involving the determination of physical
constituents like percentage of
(i) Moisture content.
(ii) Volatile matter.
(iii) Ash content.
(iv) Fixed carbon in coal.
1. Moisture content
About 1 gm of powdered air-dried coal sample is taken in a
crusible, and is heated at 100 – 105°C in an electric hot-air oven for 1 hour.
The loss in weight of the sample is found out and the % of moisture is
calculated as
% of moisture in coal = loss in weight of the coal / weight of
air-dried coal × 100

2. Volatile matter
After the analysis of moisture content the crusible with residual
coal sample is covered with a lid, and is heated at 950 ± 20°C for 7 minutes in
a muffle furnace. The loss in weight of the sample is found out and the % of
volatile matter is calculated as
% of volatile matter in coal = loss in weight of the coal / weight
of air-dried coal × 100
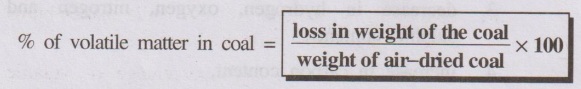
3. Ash content
After the analysis of volatile matter, the crusible with residual
coal sample is heated without lid at 700 ± 50°C for 1/2 an hour in a muffle
furnace. The loss in weight of the sample is found out and the % of ash content
is calculated as
% of ash content in coal = weight of ash formed / weight of
air-dried coal × 100
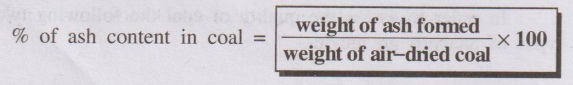
4. Fixed carbon
It is determined by subtracting the sum total of moisture,
volatile and ash contents from 100.
% of fixed carbon in coal
= 100 – % of (moisture content + volatile matter + ash content)
Significance (or) Importance of Proximate Analysis
1. Moisture content
High percentage of moisture is undesirable because
(i) it reduces the calorific value of coal,
(ii) moisture in coal consumes more heat in the form of latent
heat of evaporation and hence more heat is to be supplied to the coal,
(iii) it increases the transport cost.
2. Volatile matter
High percentage of volatile matter is undesirable because
(i) it reduces the calorific value of coal,
(ii) large proportion of fuel on heating will distill over as
vapour, which escapes out unburnt,
(iii) coal with high percentage of volatile matter burns with a
long flame with high smoke,
(iv) the coal containing high percentage of volatile matter do not
coke well.
3. Ash content
High percentage of ash content is undesirable because
(i) it reduces the calorific value of coal,
(ii) ash causes hindrance to heat flow as well as produces
clinkers, which blocks the air supply through the fuel,
(iii) it increases the
transporting, handling and storage costs,
(iv) it involves additional cost in ash disposal.
4. Fixed carbon
(i) High percentage of fixed carbon is desirable because higher
the percentage of fixed carbon in a coal, greater is its calorific value,
(ii) the percentage of fixed carbon helps in designing the furnace
and the shape of the fire-box.
2. Ultimate Analysis
It is the analysis involving the determination of chemical
constituents like percentage of
(i) carbon and hydrogen contents
(ii) nitrogen content
(iii) sulphur content
(iv) ash content
(v) oxygen content
1. Carbon and Hydrogen contents
A known amount of the coal sample is burnt in a current of O2
in a combustion apparatus. The carbon and hydrogen, present in the coal sample,
are converted into CO2 and H2O respectively according to
the following equations.
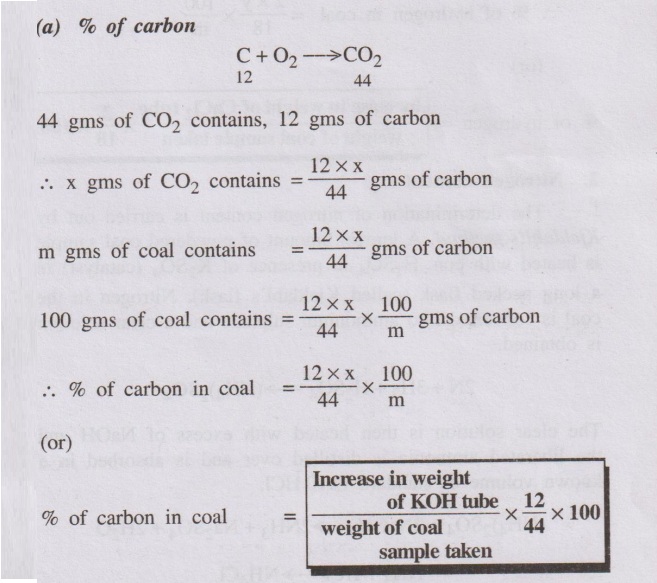
C + O2 → CO2
↑
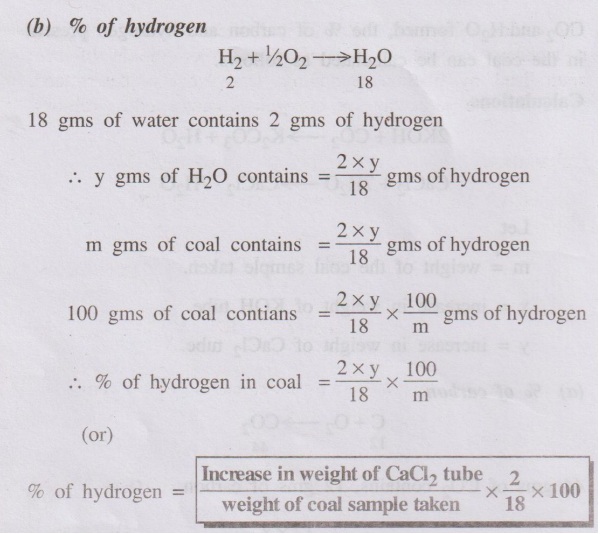
H2 + 1/2O2 → H2O ↑
The liberated CO2 and H20 vapours are absorbed respectively in KOH
and anhydrous CaCl2 tubes of known weights. The increase in weight
of KOH tube is due to the formation of CO2 while increase in weight
of CaCl2 tube is due to the formation of H2O. From the
weights of CO2 and H2O formed, the % of carbon and
hydrogen present n the coal can be calculated as follows.
Calculations
2KOH + CO2 → K2CO3 + H2O
CaCl2 + 7H2O → CaCl2 . 7H2O
Let
m = weight of the coal sample taken.
x = increase in weight of KOH tube.
y = increase in weight of CaCl2 tube.
(a) % of carbon
(b) % of hydrogen
2. Nitrogen content
The determination of nitrogen content is carried out by Kjeldahl’s
method. A known amount of powdered coal sample is heated with con. H2SO4
in presence of K2SO4 (catalyst) in a long necked flask
(called Kjeldahl's flask). Nitrogen in the coal is converted into ammonium
sulphate and a clear solution is obtained
2N + 3H2 + H2SO4 → (NH4)2SO4
The clear solution is then heated with excess of NaOH and the
liberated ammonia is distilled over and is absorbed in a known volume of
standard N/10 HCl.
(NH4)2SO4 + 2NaOH → 2NH3
+ Na2SO4 + 2H2O
NH3 + HCI → NH4Cl
The volume of unused N/10 HCl is then determined by titrating it
against standard N/10 NaOH. Thus the amount of acid neutralised by liberated
ammonia from coal is determined. From this the percentage of nitrogen is
calculated as follows.
Calculation
Let, the weight of the coal sample taken = m gms
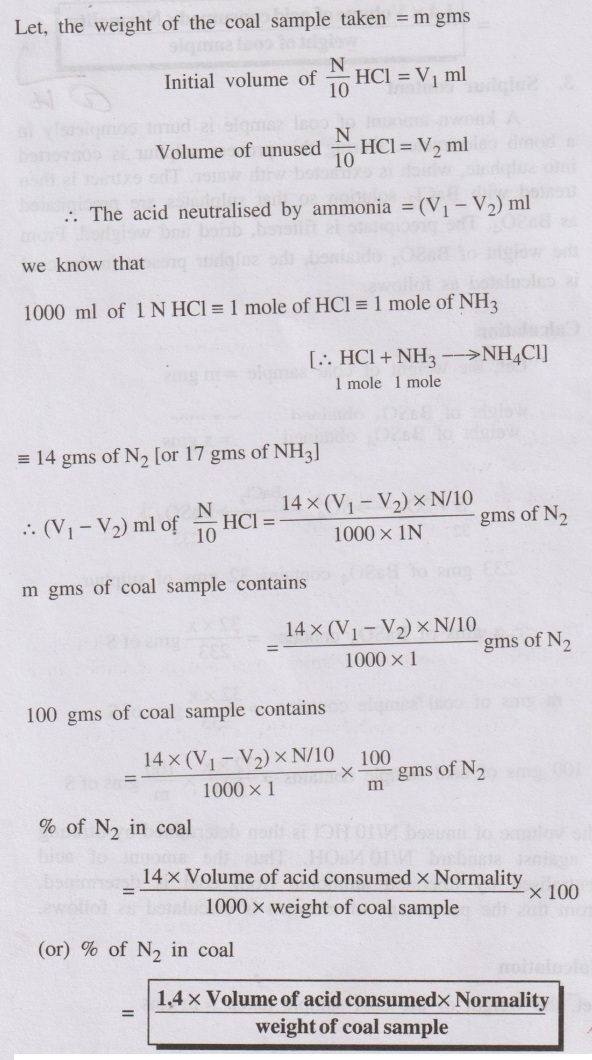
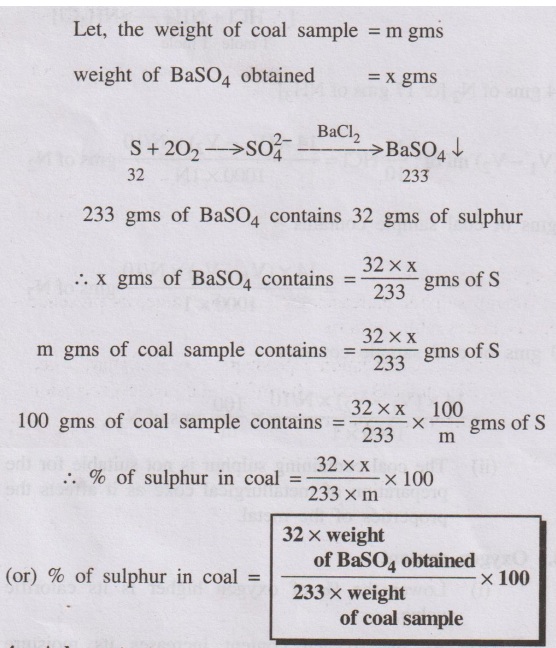
3. Sulphur content
A known amount of coal sample is burnt completely in a bomb
calorimeter. During this process sulphur is converted into sulphate, which is
extracted with water. The extract is then treated with BaCl2
solution so that sulphates are precipitated as BaSO4. The
precipitate is filtered, dried and weighed. From the weight of BaSO4 obtained,
the sulphur present in the coal is calculated as follows.
Calculation
Let, the weight of coal sample = m gms
weight of BaSO4 obtained = x gms
4. Ash content
Determination of ash content is carried out as in proximate
analysis
5. Oxygen content
The percentage of oxygen is calculated as follows.
% of oxygen in coal = 100 –
% of (C+H+N+S+ash)
Significance (or) Importance of Ultimate Analysis
1. Carbon and hydrogen contents
(i) Higher the % of carbon and hydrogen, better is the quality of
coal and higher is its calorific value.
(ii) The % of carbon is helpful in the classification of coal.
(iii) Higher % of carbon in coal reduces the size of combustion
chamber required.
2. Nitrogen content
(i) Nitrogen does not have any calorific value, and its presence
in coal is undesirable.
(ii) Good quality coal should have very little nitrogen content.
3. Sulphur content
Though sulphur increases the calorific value, its presence in coal
is undesirable because
(i) The combustion products of sulphur, i.e., SO2 and
SO3 are harmful and have corrosion effects on equipments.
(ii) The coal containing sulphur is not suitable for the
preparation of metallurgical coke as it affects the properties of the metal.
4. Oxygen content
(i) Lower the % of oxygen higher is its calorific value.
(ii) As the oxygen content increases its moisture holding capacity
increases, and the calorific value of the fuel is reduced.
Table 5.1 Differences between proximate analysis and ultimate
analysis
Proximate analysis
1. It involves the determinations of physical constituents like
moisture, volatile, ash and fixed carbon contents in coal.
2. It gives the approximate composition of the main constituents
of coal.
Ultimate analysis
1. It involves the determination of chemical constituents
like carbon, hydrogen, nitrogen and sulphur and oxygen contents in coal.
2. It gives the exact composition of the elementary constituents
of coal.
Engineering Chemistry: Unit IV: a. Fuels : Tag: Engineering Chemistry : - Analysis of Coal
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
