Engineering Chemistry: Unit IV: b. Combustion of Fuels
Anna University 2 Marks Questions and Answers
Combustion of Fuels | Engineering Chemistry
Engineering Chemistry : UNIT IV : Fuels and conbustion : Anna University Two Marks Questions & Answers
Anna University TWO MARKS Questions & Answers
6. Combustion of Fuels
1. What is meant by combustion of fuels?
Combustion is a process of rapid exothermic oxidation, in which a
fuel burns in the presence of oxygen with the evolution of heat and light.
2. Mention combustible and non-combustible constituents present in
the fuel.
Combustible constituents : C, H, S. and O
Non-combustible constituents : N, CO2
3. Define calorific value of a fuel. (TNV A.U.T. July 2010)
(or)
What is meant by calorific value of fuel. (A.U Dec 2015, June 2016)
The calorific value of a fuel is defined as "the total amount
of heat liberated, when a unit mass of fuel is burnt completely.”
4. Define GCV and LCV of a fuel. (A.U. Dec 2005, June 2006)
(i) Higher (or) Gross calorific value (G.C.V)
It is defined as the total amount of heat produced, when a unit
quantity of the fuel is completely burnt and the products of combustion are
cooled to room temperature.
(ii) Lower (or) Net Calorific Value (N.C.V)
It is defined as the net heat produced, when a unit quantity of
the fuel is completely burnt and the products of combustion are allowed to
escape.
5. What is Calorie. (A.U. Jan.
2018)
It is defined as the amount of heat required to raise the
temperature of 1 gram of water through 1°C (15 to 16°C).
6. Name the important units of calorific values. (A.U Jan. 2018)
(i) Calorie
(ii) Kilocalorie
(iii) British Thermal Unit (B.T.U)
(iv) Centigrade Heat Unit (C.H.U).
7. What are the factors, which influence the rate of combustion?
The rate of combustion depends on the following factors
1. The nature of the combustible substance (fuel).
2. The temperature.
3. The concentration of the fuel and air.
8. Give the Dulong's formula for the calculation of GCV and NCV.
GCV = 1/100 [8050 C + 34500
(H – O/8) + 2240 S ] k. cal/kg
NCV = [HCV – 9/100 H × 587] k. cal/kg.
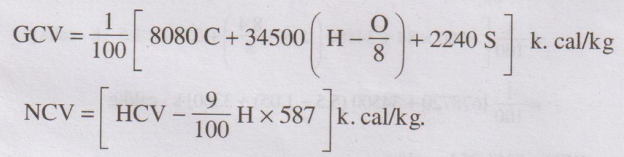
9. What are the reagents used in flue gas analysis? Indicate their
functions?
(or)
Name the reagents used for absorbing CO2, CO and O2
during flue gas analysis by orsat apparatus.
Reagents : Absorbent
KOH solution : CO2
Alkaline pyrogallic acid : O2
Ammoniacal cuprous chloride : CO

10. Mention the uses of flue gas analysis.
Flue gas analysis gives an idea about the complete or incomplete
combustion process. If the flue gases contain considerable amount of CO, it
indicates that incomplete combustion. If the flue gases contain considerable
amount of O2, it indicates that complete combustion.
11. What is the molecular mass and density of air?
Molecular mass of air is taken as 28.94 g/mol
Density of air at NTP = 1.29 kg/m3
12. The ultimate analysis of a coal sample indicates Carbon = 85,
Sulphur = 1.5%, Nitrogen = 0.6%, Hydrogen = 5.5% and Oxygen = 8.4%. Calculate
the GCV.(A.U. June 2012)
Solution
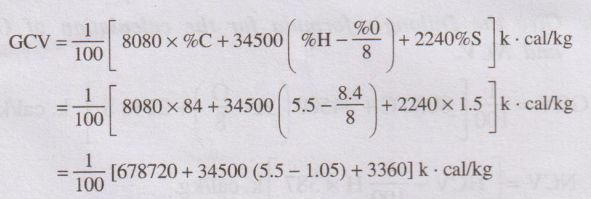
13. What is ignition temperature? (AU June 2014)
It is “the lowest temperature to which the fuel must be heated, so
that it starts burning smoothly”.
14. Define spontaneous ignition temperature. (AU June 2014)
It is defined as “the minimum temperature at which the fuel
catches fire (ignites) spontaneously without external heating”.
15. Define explosive range of a fuel. (AU June 2014)
All gaseous fuels have two limits called upper limit and lower
limit. These limits represents percentage by volume of fuel present in fuel-air
mixture.
(i) Lower limit represents the smallest proportion of combustible
gas (fuel).
(ii) Upper limit represents the largest proportion of combustible
gas.
The range covered by these limits is termed as explosive range of
the fuels.
16. Define carbon emission?
It is defined as the release of carbon into the atmosphere. Since
green house gas emissions are often calculated as carbon dioxide equivalents,
they are often referred to as “carbon emissions”.
17. Suggest any two methods of reducing carbon emission.
Carbon emission can be reduced by reducing green house gas
emission. It can be done by the following ways. 1. In industry, green house
gases can be reduced by many ways.
(i) Including energy efficiency
(ii) Fuel switching
(iii) Combined heat and power
(iv) Use of renewable energy
2. Avoid of using HFC's in refrigeration, air conditioning and
foam blowing.
18. Define carbon footprint.
It is the total amount of green house gases (including CO2
and CH4) that are generated (emitted) by our direct and indirect
activities.
Engineering Chemistry: Unit IV: b. Combustion of Fuels : Tag: Engineering Chemistry : Combustion of Fuels | Engineering Chemistry - Anna University 2 Marks Questions and Answers
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
