Engineering Chemistry: Unit IV: a. Fuels
Anna University Long Questions and Answers
Fuels | Engineering Chemistry
Engineering Chemistry : UNIT IV : Fuels and conbustion : Anna University long Questions & Answers
Unit - IV
Chapter 5
Fuels
Anna University Long Questions & Answers
Part - B
1. What is meant by proximate analysis of coal? What are the
quantities estimated in this analysis and their significance? (A.U May 2015)
(or)
What is proximate analysis? Write its significance. (Chen A.U. May 2009)
(or)
What is meant by proximate analysis. (A.U July 2016, Dec 2015)
(i) Proximate analysis
Proximate analysis is the analysis involving the determination of
the following physical constituents.
(ii) Determination
(a) Moisture content
About 1 gm of powdered air-dried coal sample is taken in a
crusible, and is heated at 100 – 105°C in an electric hot-air oven for 1 hour.
The loss in weight of the sample is found out and the % of moisture is
calculated as
% of moisture in coal = loss
in weight of the coal / weight of air-dried coal × 100
(b) Volatile matter
After the analysis of moisture content the crusible with residual
coal sample is covered with a lid, and is heated at 950 – 20°C for 7 minutes in
a muffle furnace. The loss in weight of the sample is found out and the % of
volatile matter is calculated as
% of volatile matter in coal = loss in weight of the coal / weight
of air-dried coal × 100
(c) Ash content
After the analysis of volatile matter, the crusible with residual
coal sample is heated without lid at 700 + 50°C for 1/2 an hour in a muffle
furnace. The loss in weight of the sample is found out and the % of ash content
is calculated as
% of ash content in coal = weight of ash formed / weight of
air-dried coal × 100
(d) Fixed carbon
It is determined by subtracting the sum total of moisture,
volatile and ash contents from 100.
% of fixed carbon in coal = 100 – % of (moisture content +
volatile matter + ash content)
Significance (or) Importance of Proximate Analysis
(a) Moisture content
High percentage of moisture is undesirable because it reduces the
calorific value of coal,
(b) Volatile matter
High percentage of volatile matter is undesirable because it
reduces the calorific value of coal,
(c) Ash content
High percentage of ash content is undesirable because it reduces
the calorific value of coal,
(d) Fixed carbon
High percentage of fixed carbon produces higher calorific value.
2. Discuss the ultimate analysis of coal. (TCY A.U. Dec 2009) (A.U. Dec 2009, May 2017)
(or)
Describe the ultimate analysis of coal. (A.U. June 2014)
It involves the determination of the followings
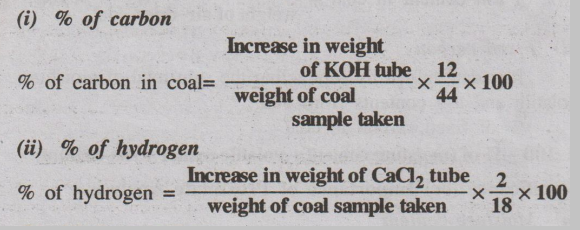
(a) Carbon and Hydrogen contents
A known amount of the coal sample is burnt in a current of O2
in a combustion apparatus. The carbon and hydrogen, present in the coal sample,
are converted into CO2 and H2O respectively according to
the following equations.
C + O2 → CO2
↑
H2 + 1/2O2 → H2O↑
The liberated CO2 and H2O vapours are
absorbed respectively in KOH and anhydrous CaCl2 tubes of known
weights.
(i) % of carbon
(ii) % of hydrogen
(b) Nitrogen content
..The determination of nitrogen content is carried out by Kjeldahl's
method. A known amount of powdered coal sample is heated with con. H2SO4
in presence of K2SO4 (catalyst) in a long necked flask
(called Kjeldahl's flask). Nitrogen in the coal is converted into ammonium
sulphate and a clear solution is obtained.
The clear solution is then heated with excess of NaOH and the
liberated ammonia is absorbed in a known volume of standard N/10 HCI.
The volume of unused N/10 HCl is then determined by titrating it
against standard N/10 NaOH. Thus the amount of acid neutralised by liberated
ammonia from coal is determined. From this the percentage of nitrogen is
calculated as follows. % of N2 in coal
= 14 × Volume of acid consumed × Normality / 1000 × weight of coal
sample × 100
(c) Sulphur content
A known amount of coal sample is burnt completely in a bomb
calorimeter. During this process sulphur is converted into sulphate, which is
extracted with water. The extract is then treated with Bal2 solution
so that sulphates are precipitated as BaSO4. The precipitate is
filtered, dried and weighed. From the weight of BaSO4 obtained, the
sulphur present in the coal is calculated as follows.
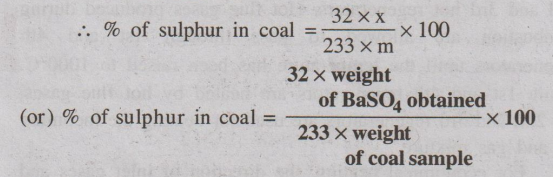
(d) Ash content
Determination of ash content is carried out as in proximate
analysis
(e) Oxygen content
The percentage of oxygen is calculated as follows.
% of oxygen in coal = 100 – % of (C + H + N + S + ash)
3. With neat diagram, explain the manufacturing of metallurgical
coke by Otto-Hoffman method. (A.U Dec
2014, June 2016)
(or)
Describe the Otto-Hoffman method of coke manufacture and the
recovery of various by product. (Coim A.U. July 2009)(Dec 2009)(TNV A.U. May 2009) (May
2008, Dec 2012, Dec 2015)
The oven consists of a number of silica chambers. Each chamber is
provided with a charging hole at the top.
Coal is introduced into the silica chamber and the chambers are
closed. The chambers are heated to 1200°C by burning the preheated air and the
producer gas mixture in the interspaces between the chambers.
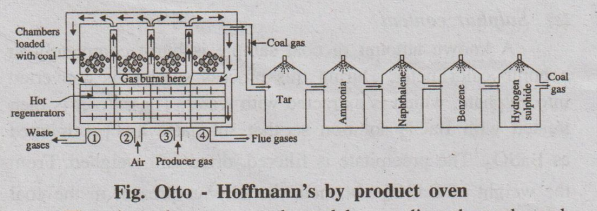
Fig. Otto - Hoffmann's by product oven
The air and gas are preheated by sending them through 2nd and 3rd
hot regenerators. Hot flue gases produced during combustion are allowed to pass
through 1st and 4th regenerators until the temperature has been raised to
1000°C. While 1st and 4th regenerators are heated by hot flue gases, the 2nd
and 3rd regenerators are used for heating the incoming air and gas mixture.
. For economical heating, the direction of inlet gases and flue
gases are changed frequently. The above system of recycling the flue gases to
produce heat energy is known as the regenerative system of heat economy.
When the process is complete, the coke is removed and quenched with water.
Time taken for complete carbonisation is about 12-20 hours. The
yield of coke is about 70%.
The valuable by products like coal gas, tar, ammonia, H2S and
benzol, etc. can be recovered from flue gas.
Recovery of by-products
(i) Tar: The coal gases are first passed through a tower in which liquor
ammonia is sprayed. Tar and dust get dissolved and collected in a tank below,
which is heated by steam coils to recover back the ammonia sprayed.
(ii) Ammonia: The gases are then passed
through another tower in which water is sprayed. Here ammonia gets converted to
NH OH.
(iii) Naphthalene: The gases
are again passed through a tower, in which cooled water is sprayed. Here
naphthalene gets condensed.
(iv) Benzene: The gases are passed
through another tower, where petroleum is sprayed. Here benzene gets condensed
to liquid.
(v) Hydrogen Sulphide: The
remaining gases are then passed through a purifier packed with moist Fe2O3.
Here H2S is retained.
The final gas left out is called pure coal gas which is used as a
gaseous fuel.
4. What are the characteristics of good metallurgical coke? (A.U May 2007) (i)
Purity: The moisture, ash, sulphur
and phosphorus contents in metallurgical coke should be low, because moisture
and ash reduce the calorific value. Sulphur and phosphorus may contaminate the
metal.
(ii) Porosity: Coke should be highly
porous so that oxygen will have intimate contact with carbon and combustion
will be complete and uniform.
(iii) Strength: The coke
should have very high mechanical strength inorder to withstand high pressure of
the overlying material in the furnace.
(iv) Calorific value: The
calorific value of coke should be very high.
(v) Combustibility: The coke
should burn easily.
(vi) Reactivity: The
reactivity of the coke should be low because low reactive cokes produce high
temperature on combustion.
(vii) Cost: It should be cheap and
readily available.
5. Explain the petroleum refinary process in detail with neat
sketches. What are the properties and applications of its various fractions. (AU June 2013)
The process of refining involves the following steps.
Step 1: Separation of water (Cottrell's process)
The crude oil from oil well is an extremely stable emulsion of oil
and- salt water. The crude oil is allowed to flow between two highly charged
electrodes, where colloidal water droplets combine to form large drops, which
is then separated out from the oil.
Step 2: Removal of harmful sulphur compounds
Sulphur compounds are removed by treating the crude oil with
copper oxide. The copper sulphide formed is separated out by filtration.
Step 3: Fractional distillation
The purified crude oil is then heated to about 400°C in an iron
retort, where the oil gets vapourised. The hot vapours are then passed into the
bottom of a “fractionating column" (Fig :). The fractionating column is a
tall cylindrical tower containing a number of horizontal stainless steel trays
at short distances. Each tray is provided with small chimney covered with a
loose cap.
When the vapours of the oil go up in the fractionating column,
they become cooler and get condensed at different trays. The fractions having
higher boiling points condense at lower trays whereas the fractions having
lower boiling points condense at higher trays.
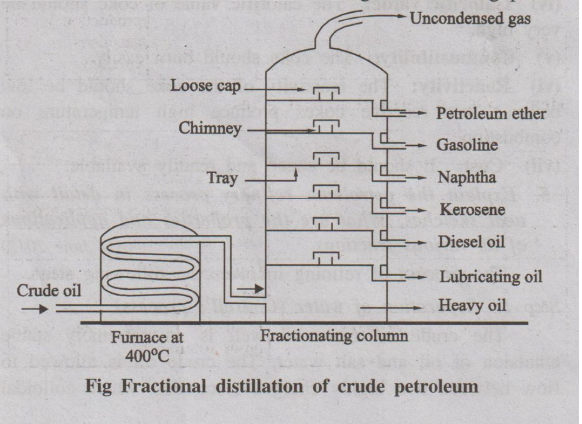
Fig Fractional distillation of crude petroleum
The gasoline obtained by this fractional distillation is called
straight-run gasoline. Various fractions obtained at different trays are given
in table.
Table : Various fractions, compositions and their uses
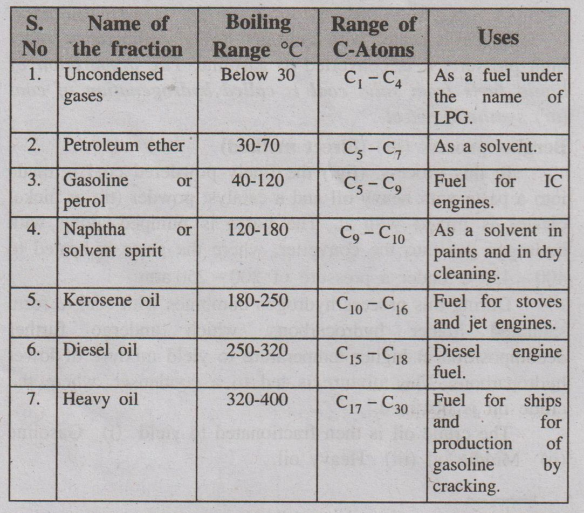
Heavy oils on refractionation gives
Name of the Fraction : Uses
1. Lubricating oils : As lubricants.
2. Petroleum jelly or vaseline : Used in medicines and cosmetics.
3. Grease : Used as lubricant.
4. Paraffin wax : Used in candles, boot polishes etc.
Pitch at above 400°C : Used for making roads, water proof roofing
etc.
6. What is synthetic petrol? How is it manufactured by Bergius process? (TCY A.U. Dec 2009) (TNV A.U May 2009)(Chen A.U. May 2009)
(or)
Describe the manufacture of petrol by Bergius process. (A.U. June 2014)
If coal is heated with hydrogen to high temperature under high
pressure, it is converted to gasoline. The preparation of liquid fuels
from solid coal is called hydrogenation of coal (or) synthetic petrol.
Bergius process (or) (direct method)
In this process, (fig.) the finely powdered coal is made into a
paste with heavy oil and a catalyst powder (tin or nickel oleate) is mixed with
it. The paste is pumped along with hydrogen gas into the converter, where the
paste is heated to 400 – 450°C under a pressure of 200 – 250 atm.
During this process hydrogen combines with coal to form saturated
higher hydrocarbons, which undergo further decomposition at higher temperature
to yield mixture of lower hydrocarbons. The mixture is led to a condenser,
where the crude oil is obtained.
The crude oil is then fractionated to yield (i) Gasoline (ii)
Middle oil (iii) Heavy oil.
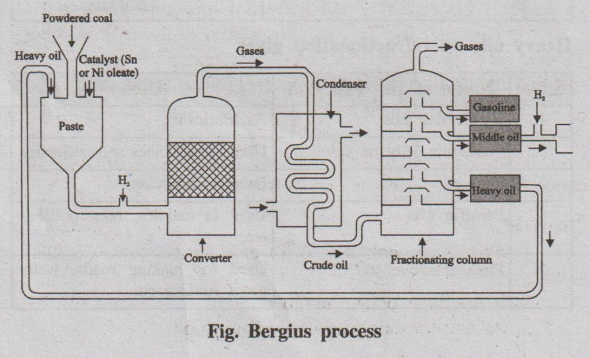
Fig. Bergius process
The middle oil is further hydrogenated in vapour phase to yield
more gasoline. The heavy oil is recycled for making paste with fresh coal dust.
The yield of gasoline is about 60% of the coal used.
7. What is power alcohol? Explain its manufacture and properties. (A.U Dec 2015)
(Or)
Write notes on power alcohol (A.U.
Dec 2014)
When ethyl alcohol is blended with petrol at. concentration of
5-10%, it is called power alcohol.
Manufacture
Manufacture of power alcohol involves the following two steps
Step 1: Manufacture of Ethyl alcohol
Ethyl alcohol can be synthesised by fermentation of carbohydrates
in presence of yeast. This fermentation yields only about 20% alcohol.
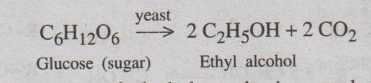
Concentration of alcohol can be increased up to 97.6% by
fractional distillation yields rectified spirit.
Step II: Conversion of ethyl alcohol into power alcohol
But, for the use in IC engines, 100% alcohol (absolute alcohol) is
prepared by removing last traces of water from rectified spirit. It can be done
by the following method.
Alcohol is distilled in the presence of dehydrating agent, which
holds the water.
Finally absolute alcohol is mixed with petrol at concentration of
5-10% to get power alcohols.
Properties
1. Power alcohol has a lower calorific values (7000 k.cal/kg).
2. It has high octane number (90).
3. Its anti-knocking properties are good.
4. It generates 10% more power than the gasoline of same quantity.
5. Its compression ratio is also higher.
8. What is bio-diesel? Explain trans-esterification and advantages
of bio-diesel. (A.U. May 2017)
Fuel that is made from natural elements such as plants, vegetables
is called bio-diesel.
(Or)
Bio-diesel is a vegetable oil based diesel fuel, consisting of
long-chain alkylester.
Manufacture : Trans-esterification (or) alcoholysis
It involves treatment of vegetable oil (sunflower oil, palm oil,
soyabean oil, mustard oil, etc) with excess of methanol in presence of catalyst
to give mono ethyl esters of long chain fatty acid and glycerine. It is allowed
to stand for some time and glycerine is separated.
Alcoholysis reaction is represented as
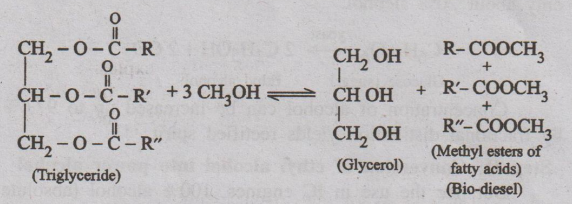
Methyl esters of fatty acids, thus formed, are called
“Bio-diesel”. Bio-diesel is a pure fuel before blending with conventional
diesel fuel. Bio-diesel can be blended with petroleum diesel.
Advantages
1. Bio-diesel is bio-degradable.
2. It is prepared from renewable resources.
3. The gaseous pollutants are lesser as compared to the conventional
diesel fuel.
4. Bio-diesel can be produced from different types of vegetable
oils.
5. Best engine performance and less smoke emission are achieved.
9. What is meant by knocking in petrol engines? How is knocking
prevented? (A.U May 2015)
Definition
Knocking is a kind of explosion due to rapid pressure rise
occurring in an IC engine.
In a petrol engine, a mixture of gasoline vapour and air at 1:17
ratio is used as fuel.
This mixture is compressed and ignited by an electric spark. The
products of oxidation reaction (combustion) increases the pressure and pushes
the piston down the cylinder.
If the combustion proceeds in a regular way, there is no problem
in knocking. But in some cases, the rate of combustion (oxidation) will not be
uniform due to unwanted chemical constituents of gasoline. The rate of ignition
of the fuel gradually increases and the final portion of the fuel-air mixture
gets ignited instantaneously producing an explosive sound known as “Knocking”.
Knocking property of the fuel reduces the efficiency of engine. So a good
gasoline should resist knocking
Prevention of Knocking
1. By using petrol of high octane value.
2. Blending petrol of high octane number with petrol of low octane
number, so that the octane number of the latter can be improved,
3. Addition of anti-knock agents like Tetra-Ethyl Lead (TEL).
10. What is metallurgical coke? How is it superior than coal?
Describe any one method of manufacturing metallurgical coke. (A.U May 2015)
(i) Metallurgical
When bituminous coal is heated strongly in the absence of air, the
volatile matter escapes out and the mass becomes hard, strong, porous and
coherent which is called metallurgical Coke.
(or)
The coke used in the metallurgical purpose is called metallurgical
coke.
(ii) Coke is superior to coal
Coke is superior to coal because
(i) Percentage of fixed carbon and hence the calorific value of
coke is high.
(ii) Percentage of moisture, volatile and ash contents are higher
in coal, where as they are low in coke.
(iii) Manfacture of metallurgical coke
Refer Annexure Q.No.3; Page E4 - E6.
The oven consists of a number of silica chambers. Each chamber is
provided with a charging hole at the top.
Coal is introduced into the silica chamber and the chambers are
closed. The chambers are heated to 1200°C by burning the preheated air and the
producer gas mixture in the interspaces between the chambers.

Fig. Otto - Hoffmann's by product oven
The air and gas are preheated by sending them through 2nd and 3rd
hot regenerators. Hot flue gases produced during combustion are allowed to pass
through 1st and 4th regenerators until the temperature has been raised to
1000°C. While 1st and 4th regenerators are heated by hot flue gases, the 2nd
and 3rd regenerators are used for heating the incoming air and gas mixture.
. For economical heating, the direction of inlet gases and flue
gases are changed frequently. The above system of recycling the flue gases to
produce heat energy is known as the regenerative system of heat economy.
When the process is complete, the coke is removed and quenched with water.
Time taken for complete carbonisation is about 12-20 hours. The
yield of coke is about 70%.
The valuable by products like coal gas, tar, ammonia, H2S and
benzol, etc. can be recovered from flue gas.
Recovery of by-products
(i) Tar: The coal gases are first passed through a tower in which liquor
ammonia is sprayed. Tar and dust get dissolved and collected in a tank below,
which is heated by steam coils to recover back the ammonia sprayed.
(ii) Ammonia: The gases are then
passed through another tower in which water is sprayed. Here ammonia gets
converted to NH OH.
(iii) Naphthalene: The gases
are again passed through a tower, in which cooled water is sprayed. Here
naphthalene gets condensed.
(iv) Benzene: The gases are passed through
another tower, where petroleum is sprayed. Here benzene gets condensed to
liquid.
(v) Hydrogen Sulphide: The
remaining gases are then passed through a purifier packed with moist Fe2O3.
Here H2S is retained.
The final gas left out is called pure coal gas which is used as a
gaseous fuel.
Engineering Chemistry: Unit IV: a. Fuels : Tag: Engineering Chemistry : Fuels | Engineering Chemistry - Anna University Long Questions and Answers
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
