Engineering Chemistry: Unit IV: b. Combustion of Fuels
Anna University Long Questions and Answers
Combustion of Fuels | Engineering Chemistry
Engineering Chemistry : UNIT IV : Fuels and conbustion : Anna University long Questions & Answers
Unit - IV
Chapter 6
Combustion of Fuels
Anna University Long Questions & Answers
Part - B
1. Explain: Gross and Net calorific value. (A.U. Dec 06)
(a) Higher (or) Gross calorific value (GCV)
It is defined as the total amount of heat produced, when a
unit quantity of the fuel is completely burnt and the products of combustion
are cooled to room temperature.
When a fuel containing hydrogen is burnt, the hydrogen is
converted into steam. If the combustion products are cooled to room
temperature, the steam gets condensed into water and latent heat is evolved.
Thus, the latent heat of condensation of steam is also included in gross
calorific value.
(b) Lower (or) Net Calorific Value (NCV)
It is defined as the net heat produced, when a unit quantity
of the fuel is completely burnt and the products of combustion are allowed to
escape.
ஃ NCV = GCV - Latent heat of condensation of water vapour produced.
= GCV - Mass of hydrogen × 9 × Latent heat of condensation of
water vapour.
1 part by weight of H2 produces 9 parts by weight of H2O
as follows. The latent heat of steam is 587 cal/gm.
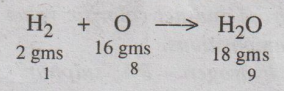
Thus,
NCV = GCV – (9/100) H × 587
= GCV - 0.09H × 587
where
H = % of H2 in the fuel.
2. Define gross and net calorific values. Calculate gross and net
calorific values of a coal sample containing 84% carbon, 1.5% sulphur, 6%
nitrogen, 5.5% hydrogen and 8.4% oxygen. (A.U
May 2015)
(i) GCV & NCV
Refer Annexure Q.No.1; Page 61
(ii) Problem
Solution
(i) Gross calorific value (GCV)
(ü) Net Calorific Value (NCV)
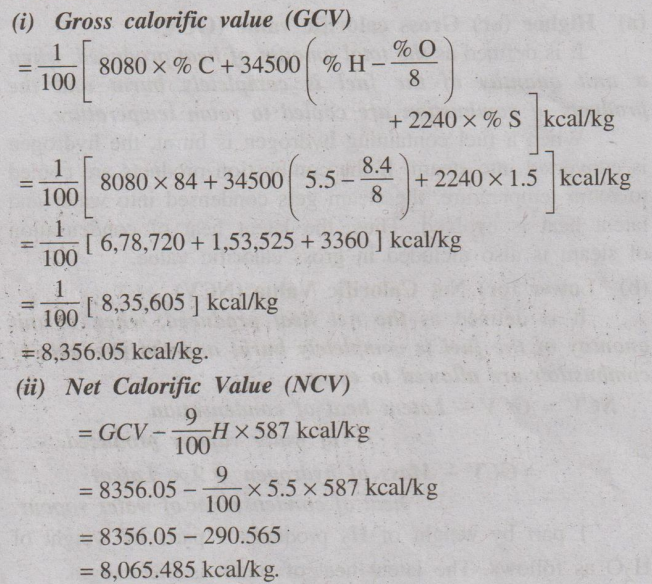
3. Calculate the gross and net calorific value of coal having the
following compositions.
Carbon - 85%, hydrogen - 8%, sulphur - 1%, nitrogen - 2% and ash -
4% (A.U. May 2007)
Solution
(i) Gross calorific value
(GCV)
= 1 / 100 [8080 × % C + 34500 (% H - %O / 8) + 2240 × % S] kcal/kg
= 1 / 100 [8080 × 85 + 34500 (8 – 0/8 ) + 2240 × 1] kcal/kg
= 1/ 100 [6,86,800 +
2,76,000 + 2240 ] kcal/kg
= 1 / 100 [ 9,65,040 ] kcal/kg
= 9,650.4 kcal/kg.
(ii) Net Calorific Value (NCV)
= GCV – (9 / 100) H × 587
kcal/kg
= 9650.4 – (9 / 100) × 8 ×
587 kcal/kg
= 9650.4 – 422.64
= 9227.76 kcal/kg.
4. With a neat diagram explain the analysis of flue gas by Orsat
apparatus and mention the precautions to be followed during the analysis. (Chen A.U. Dec 2009 July 2016)
(or)
Explain flue gas analysis by ORSAT method. Give suitable diagram. (A.U. June
2014, Dec 2014, May 2017)
Description of orsat's apparatus
It consists of a horizontal tube. At one end of this tube, U-tube
containing fused CaCl2 is connected through 3-way stop cock. The
other end of this tube is connected with a graduated burette. The burette is
surrounded by a water-jacket to keep the temperature of gas constant. The lower
end of the burette is connected to a water reservoir by means of a rubber tube.
The level of water in the burette can be raised or lowered by raising or
lowering the reservoir (fig ) The horizontal tube is also connected with three
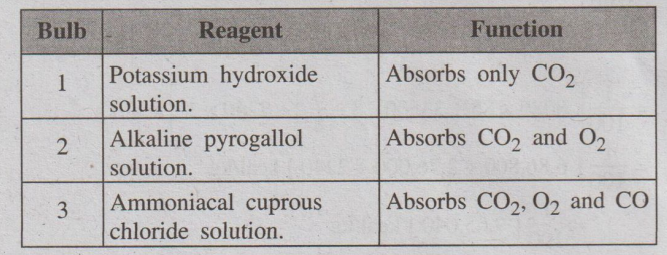
different absorption bulbs 1, 2, and 3 for absorbing CO2, O2
and CO.
Working
The 3-way stop-cock is opened to the atmosphere and the reservoir
is raised, till the burette is completely filled with water and air is excluded
from the burette. The 3-way stop-cock is now connected to the flue gas supply
and the flue gas is sucked into the burette and the volume of flue gas is
adjused to 100 cc by raising and lowering the reservoir. Then the 3-way stop
cock is closed.
(a) Absorption of CO2
The stopper of the absorption bulb-1, containing KOH solution, is
opened and all the gas is passed into the bulb-1 by raising the level of water
in the burette. The gas enters into the bulb-1, where CO2 present in
the flue gas is absorbed by KOH.
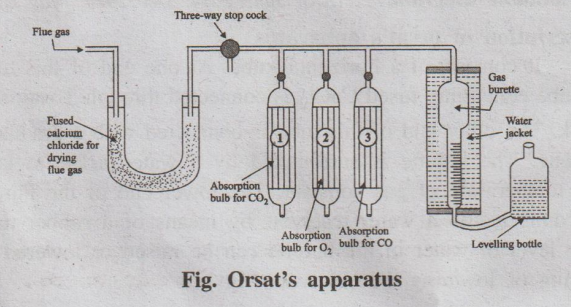
Fig. Orsat's apparatus
The gas is again sent to the burette. This process is repeated
several times to ensure complete absorption of CO2, The decrease in
volume of the flue gas in the burette indicates the volume of CO2 in
100 cc of the flue gas.
(b) Absorption of O2
Stop-cock of bulb-1 is closed and stop cock of bulb-2 is opened.
The gas is again sent into the absorption bulb-2, where O2
present in the flue gas is absorbed by alkaline pyrogallol. The decrease in
volume of the flue gas in the burette indicates the volume of O2.
(c) Absorption of Co
Now stop-cock of bulb-2 is closed and stop-cock of bulb-3 is
opened. The remaining gas is sent into the absorption bulb-3, where CO present
in the flue gas is absorbed by ammoniacal cuprous chloride. The decrease in
volume of the flue gas in the burette indicates the volume of Co. The remaining
gas in the burette after the absorption of CO2, O2,& CO
is taken as nitrogen.
Precautions
1. Care must be taken in such a way that, the reagents in the
absorption bulb 1, 2 and 3 should be brought to the etched marked level one by
one by raising and lowering reservoir bottle.
2. All the air from the reservoir bottle is expelled to atmosphere
by lifting the reservoir bottle.
3. It is essential that CO2, O2 and CO are
absorbed in that order only
4. As the CO content in flue gas is very small, it should be
measured quite carefully.
5. Write notes on (i) IT & SIT (ii) Explosive range (i)
(a) Ignition Temperature (IT)
It is defined as, "the lowest temperature to which the
fuel must be heated, so that it starts burning smoothly".
Ignition temperature of coal is about 300°C. In the case of liquid
fuels, the ignition temperature is called the flash point, which ranges from
200 – 450°C. For gaseous fuels, the ignition temperature is in the order of
800°C.
(b) Spontaneous Ignition Temperature (SIT)
It is defined as "the minimum temperature at which the
fuel catches fire (ignites) spontaneously without external heating”.
If the ignition temperature of a fuel is low it can catch fire
very quickly. On the other hand if the ignition temperature is high it is
difficult to ignite the fuel. If the heat evolved in a system is unable to
escape, temperature of the system goes on increasing and when SIT is reached,
the system burns on its own.
(ii) Explosive Range (or) Limits of Inflammability
All gaseous fuels have two limits called upper limit and lower
limit. These limits represents percentage by volume of fuel present in fuel-air
mixture.
1. Lower limit represents the smallest proportion of combustible
gas (fuel).
2. Upper limit represents the largest proportion of combustible
gas.
The range covered by these limits is termed as explosive range of
the fuels. For continuous burning the amount of fuel present in the fuel-air
mixture should not go below the lower limit or above the upper limit.
Example
The explosive range of petrol is 2-4.5. This means that when the
concentration of petrol vapour in petrol-air mixture is between 2 and 4.5 by
volume, the mixture will burn on ignition. When the concentration of petrol
vapour in petrol-air mixture is below 2% (lower limit) or above 4.5% (upper
limit) by volume, the mixture will not burn on ignition.
Thus, explosive range (or) explosive limit is the limiting
composition of a gas-air mixture beyond which the mixture will not ignite and
continue to burn is called explosive range (or) explosive limit.
6. What is carbon footprint? Mention any 5 important sources? How
to lower carbon footprint?
Definition
It is the total amount of green house gases (including CO2
and CH4) that are generated (emitted) by our direct and indirect
activities.
Individual carbon footprint
It is the sum total of their direct and indirect carbon emissions
over the course of a year.
i.e., Smaller your carbon footprint : better for the future
Bigger your carbon footprint : Have bigger negative impact in
environment
The average carbon footprint for a person in united state is 16
tons. Globally, the average is closer to 4 tones. To avoid 2°C rise in global
temperatures, the average global carbon footprint per year needs to drop under
2 tons by 2050.
Fig. Carbon Footprint
1. Sources of carbon footprint
(i) Climate change.
(ii) Natural process like volcanos.
(iii) Green house gases emitted from human activities.
(iv) Pollution released by human beings doing human things.
(v) Transportation accounted for about 28% of total country.
(vi) Electricity generation accounted for about 28%
(vii) Industrial activities 22%
(viii) Heating and cooling in homes and businesses contribute 11%
How to lower carbon footprint
Lowering individual carbon footprint from 16 tons to 2 tons does
not happen over night. But, by making small changes in our action we can reduce
carbon footprint.
Once you understand where your emission comes from, you can take
steps to reduce your impact.
1. Calculate your carbon footprint.
2. Drive less.
3. Switch to an electric (or) hybrid car.
4. Travel smart. 5. Switch to renewable energy.
6. Consider solar panels.
7. Make your home more efficient.
8. Turn your thermostat just 2 degrees cooler in winter and 2
degrees warmer in summer.
9. Get energy efficient appliances.
10. Unplug electrical devices when not in use.
11. Buy locally - sourced food.
12. Start a home garden.
13. Eat less meat.
14. Don't waste water.
15. Reduce, reuse and recycle.
Engineering Chemistry: Unit IV: b. Combustion of Fuels : Tag: Engineering Chemistry : Combustion of Fuels | Engineering Chemistry - Anna University Long Questions and Answers
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
