Physics for Electrical Engineering: Unit II: a. Electrical Properties of Materials
Degenerate States
Electrical Properties of Materials
The three combinations of quantum numbers, (112), (121) and (211), which give the same eigen value but different eigen functions are called 3-fold degenerate state.
DEGENERATE
STATES
It is seen from equations (3) and
(4) that for several combinations of quantum numbers, we have the same energy
eigen value but different eigen functions. Such a state of energy levels is
called degenerate state.
The
three combinations of quantum numbers, (112), (121) and (211), which give the
same eigen value but different eigen functions are called 3-fold degenerate
state.
Non-degenerate state:
When
only one wave function corresponds to the energy eigen value, such a state is
called non-degenerate state.
Suppose
nx = 2, ny = 2, nz = 2.
Then,
E222 =12h2 / 8ma2
and 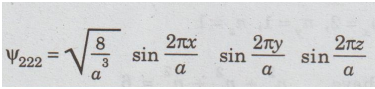
Physics for Electrical Engineering: Unit II: a. Electrical Properties of Materials : Tag: : Electrical Properties of Materials - Degenerate States
Related Topics
Related Subjects
Physics for Electrical Engineering
PH3202 2nd Semester 2021 Regulation | 2nd Semester EEE Dept 2021 Regulation
