Electromagnetic Theory: Unit II: (b) Conductors, Dielectrics and Capacitance
Dielectric Materials
• It is seen that the conductors have large number of free electrons while insulators and dielectric materials do not have free charges. The charges in dielectrics are bound by the finite forces and hence called bound charges.
Dielectric Materials
AU
; May-04,18, Dec.-06, 12, 14, 17,18, Nov.-03, 11, Dec.-17
•
It is seen that the conductors have large number of free electrons while
insulators and dielectric materials do not have free charges. The charges in
dielectrics are bound by the finite forces and hence called bound charges. As
they are bound and not free, they cannot contribute to the conduction process.
But if subjected to an electric field ![]() , they shift their relative
positions, against the normal molecular and atomic forces. This shift in the
relative positions of bound charges, allows the dielectric to store the energy.
, they shift their relative
positions, against the normal molecular and atomic forces. This shift in the
relative positions of bound charges, allows the dielectric to store the energy.
•
The shifts in positive and negative charges are in opposite directions and
under the influence of an applied electric field ![]() such charges act
like small electric dipoles.
such charges act
like small electric dipoles.
Key
Point : When the dipole results from the displacement of
the bound charges, the dielectric is said to be polarized.
•
And these electric dipoles produce an electric field which opposes the
externally applied electric field. This process, due to which separation of
bound charges results to produce electric dipoles, under the influence of
electric field ![]() , is
called polarization.
, is
called polarization.
1. Polarization
•
To understand the polarization, consider an atom of a dielectric. This consists
of a nucleus with positive charge and negative charges in the form of revolving
electrons in the orbits. The negative charge is thus considered to be in the
form of cloud of electrons. This is shown in the Fig. 5.6.1.

•
Note that ![]() applied is zero. The number of positive charges is same as
negative charges and hence atom is electrically neutral. Due to symmetry, both
positive and negative charges can be assumed to be point charges of equal
amount, coinciding at the centre. Hence there cannot exist an electric dipole.
This is called unpolarized atom.
applied is zero. The number of positive charges is same as
negative charges and hence atom is electrically neutral. Due to symmetry, both
positive and negative charges can be assumed to be point charges of equal
amount, coinciding at the centre. Hence there cannot exist an electric dipole.
This is called unpolarized atom.
•
When electric field ![]() is applied, the symmetrical distribution of
charges gets disturbed. The positive charges experience a force
is applied, the symmetrical distribution of
charges gets disturbed. The positive charges experience a force  while the negative charges experience a force
while the negative charges experience a force  in the opposite
direction.
in the opposite
direction.
•
Now there is separation between the nucleus and the centre of the electron
cloud as shown in the Fig. 5.6.2 (a). Such an atom is called polarized atom.

•
It can be seen that an electron cloud has a centre separated from the nucleus.
This forms an electric dipole. The equivalent dipole formed is shown in the
Fig. 5.6.2 (b). The dipole gets aligned with the applied field. This process is
called polarization of dielectrics.
There
are two types of dielectrics,
1.
Nonpolar and 2. Polar.
•
In nonpolar molecules, the dipole arrangement is totally absent, in absence of
electric field ![]() It results only when an externally field
It results only when an externally field ![]() is applied to it. In polar molecules, the permanent displacements between
centres of positive and negative charges exist. Thus dipole arrangements exist
without application of
is applied to it. In polar molecules, the permanent displacements between
centres of positive and negative charges exist. Thus dipole arrangements exist
without application of ![]() . But such dipoles are randomly oriented. Under
the application of
. But such dipoles are randomly oriented. Under
the application of ![]() , the dipoles experience torque and they align with
the direction of the applied field
, the dipoles experience torque and they align with
the direction of the applied field ![]() . This is called polarization of
polar molecules.
. This is called polarization of
polar molecules.
•
The examples of nonpolar molecules are hydrogen, oxygen and the rare gases. The
examples of polar molecules are water, sulphur dioxide, hydrochloric acid etc.
2. Mathematical Expression for Polarization
•
When the dipole is formed due to polarization, there exists an electric dipole
moment ![]()
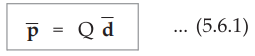
where Q = Magnitude of one of the two charges
![]() = Distance vector from negative to positive charge
= Distance vector from negative to positive charge
Let n = Number of dipoles per unit volume
Δv
= Total volume of the dielectric
N
= Total dipoles = n Δv
•
Then the total dipole moment is to be obtained using superposition principle
as,

•
If dipoles are randomly oriented,  is zero but if dipoles are aligned
in the direction of applied
is zero but if dipoles are aligned
in the direction of applied ![]() then
then  has a significant value.
has a significant value.
•
The polarization ![]() is defined as the total dipole moment per unit
volume.
is defined as the total dipole moment per unit
volume.
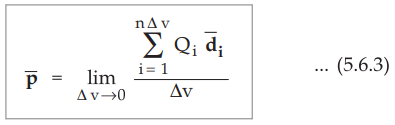
•
It is measured in coulombs per square metre (C/m2 ).
•
It can be seen that the units of polarization are same as that of flux density ![]() . Thus polarization increases the electric flux density in a
dielectric medium. Hence we can write, flux density in a dielectric is,
. Thus polarization increases the electric flux density in a
dielectric medium. Hence we can write, flux density in a dielectric is,

•
For isotropic and linear medium, the  are parallel to each other at
every point and related to each other as below
are parallel to each other at
every point and related to each other as below

where
xe = Dimensionless quantity called electric susceptibility of the material.
•
The susceptibility tells us how sensitive is a given dielectric to the applied
electric field ![]() .
.
•
Substituting (5.6.5) in (5.6.4),

•
The quantity x e +1 is defined as relative
permittivity or dielectric constant of the dielectric material.
ƐR
= Xe + 1 ... (5.6.9)
•
The medium is said to be isotropic if  are parallel i.e. in the
same direction. Thus their properties are same in all directions for isotropic
medium.
are parallel i.e. in the
same direction. Thus their properties are same in all directions for isotropic
medium.
•
Note that in anisotropic or nonisotropic materials the 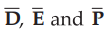 are not
parallel to each other and Ɛ and % e vary in all directions and have nine
different components. The discussion of anisotropic materials is beyond the
scope of this book.
are not
parallel to each other and Ɛ and % e vary in all directions and have nine
different components. The discussion of anisotropic materials is beyond the
scope of this book.
3. Properties of Dielectric Materials
•
The various properties of dielectric materials are,
1.
The dielectrics do not contain any free charges but contain bound charges.
2.
Bound charges are under the internal molecular and atomic forces and cannot
contribute to the conduction.
3.
When subjected to an external field ![]() , the bound charges shift their relative
positions. Due to this, small electric dipoles get induced inside the
dielectric. This is called polarization.
, the bound charges shift their relative
positions. Due to this, small electric dipoles get induced inside the
dielectric. This is called polarization.
4.
Due to the polarization, the dielectrics can store the energy.
5.
Due to the polarization, the flux density of the dielectric increases by amount
equal to the polarization.
6.
The induced dipoles produce their own electric field and align in the direction
of the applied electric field.
7.
When polarization occurs, the volume charge density is formed inside the
dielectric while the surface charge density is form ed over the surface of the
dielectric.
8.
The electric field outside and inside the dielectric gets modified due to the
induced electric dipoles.
4. Dielectric Strength
•
The ideal dielectric is nonconducting but practically no dielectric can be
ideal. As the electric field applied to dielectric increases sufficiently, due
to the force exerted on the molecules, the electrons in the dielectric become
free. Under such large electric field, the dielectric becomes conducting due to
presence of large number of free electrons. This condition of dielectric is
called dielectric breakdown. All kinds of dielectrics such as solids, liquids
and gases show the tendency of breakdown under large electric field. The
breakdown depends on the nature of material, the time and magnitude of applied
electric field and atmospheric conditions such as temperature, moisture,
humidity etc.
Key
Point : The minimum value of the applied electric field at
which the dielectric breaks down is called dielectric strength of that
dielectric.
•
The dielectric strength is measured in V/m or kV/cm. It also can be stated as
the maximum value of electric field under which a dielectric can sustain
without breakdown. Once breakdown occurs, dielectric starts conducting and no
longer behaves as dielectric. Hence all the dielectrics are assumed to be
either ideal or are not in a breakdown condition.
5. Utilization Factor
•
The utilization factor of an electric field is defined as the ratio of the
average electric field to the maximum value of an electric field. It is denoted
by 'η'.
η
= Utilization factor = Eavg / Emax
•
The reciprocal of utilization factor is called inhomogenity of an electric
field.
Ex.
5.6.1 Find the magnitude of  for a dielectric material in which |
for a dielectric material in which | ![]() | =0.15 mV/m and xe = 4.25.
| =0.15 mV/m and xe = 4.25.
Sol.
:
For a dielectric medium,

Ex.
5.6.2 A certain homogeneous slab of loss-less dielectric material is
characterized by electric susceptibility of 0.12 and carries a uniform electric
flux density inside of 1.6 nG/m2. Determine the value of
polarisation and electric field intensity.
AU
: May-04, Marks 6
Sol.
:

Ex.
5.6.3 A linear, homogeneous, isotropic dielectric material has Ɛr = 3.6 and is
covering the space between z = 0 and z = 1. If V = - 6000 z volts in the
material, find

Ex.
5.6.4 A dielectric slab of flat surface with the relative permittivity 4 is
disposed with its surface normal to a uniform field with the flux density 1.5
C/m2 . The slab occupies a volume of 0.08 m and is uniformly
polarised. Determine,
a)
The polarisation in the slab and b) The total dipole moment of slab.
AU
: Dec-14, Marks 6
Sol.
:

Examples
for Practice
Ex.
5.6.5 Find the polarization in dielectric
material with
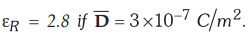
[Ans.
: 1.9285 × 10-7 C/m2]
Ex.
5.6.6 The polarization within a region having ƐR = 2.7
has the uniform value of

Review Questions
1. Explain the polarization in dielectric materials.
AU : Dec-17, May-18, Marks 6
2. Explain the properties of dielectric materials.
3. Explain in detail the behaviour of a dielectric medium in
electric field.
4. Define dielectric polarization and dielectric constant.
Electromagnetic Theory: Unit II: (b) Conductors, Dielectrics and Capacitance : Tag: : - Dielectric Materials
Related Topics
Related Subjects
Electromagnetic Theory
EE3301 3rd Semester EEE Dept | 2021 Regulation | 3rd Semester EEE Dept 2021 Regulation
