Engineering Chemistry: Unit IV: b. Combustion of Fuels
Higher and Lower Calorific Values
Definition, Calculation
1. Higher (or) Gross calorific value (GCV) 2. Lower (or) Net Calorific Value (NCV)
HIGHER AND LOWER CALORIFIC VALUES
1. Higher (or) Gross calorific value (GCV)
It is defined as the total amount of heat produced, when a
unit quantity of the fuel is completely burnt and the products of combustion
are cooled to room temperature.
When a fuel containing hydrogen is burnt, the hydrogen is
converted into steam. If the combustion products are cooled to room
temperature, the steam gets condensed into water and latent heat is evolved.
Thus, the latent heat of condensation of steam is also included in gross
calorific value.
2. Lower (or) Net Calorific Value (NCV)
It is defined as the net heat produced, when a unit quantity
of the fuel is completely burnt and the products of combustion are allowed to
escape.
ஃ NCV = GCV - Latent heat of condensation of water vapour produced.
= GCV – Mass of hydrogen × 9 × Latent heat of condensation of
water vapour.
1 part by weight of H2 produces 9 parts by weight of H2O
as follows. The latent heat of steam is 587 cal/gm.
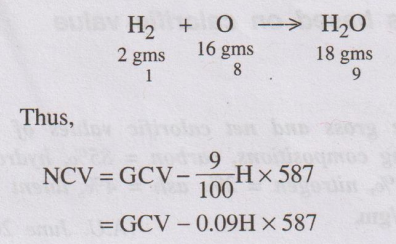
= GCV – 0.09H × 587
where
H = % of H2 in
the fuel.
Engineering Chemistry: Unit IV: b. Combustion of Fuels : Tag: Engineering Chemistry : Definition, Calculation - Higher and Lower Calorific Values
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
