Physics for Electrical Engineering: Unit II: b. Magnetic Properties of Materials
Paramagnetism in the conduction electrons in metals
Magnetic Properties of Materials
According to Langevin's theory the paramagnetic susceptibility is inversely proportional to the temperature. However, some metals have been found to paramagnetism independent of temperature.
PARAMAGNETISM
IN THE CONDUCTION ELECTRONS IN METALS
Paramagnetism of Free Electrons
According
to Langevin's theory the paramagnetic susceptibility is inversely proportional
to the temperature. However, some metals have been found to paramagnetism
independent of temperature.
It
was W. Pauli (1927) who demonstrated that this is due to paramagnetism of free
electrons (that constitute the electron gas), since they can orient only in two
directions, either along the magnetic field or against it.
In
order to understand the existence of Pauli paramagnetism, let us recall the
curve between density of states versus energy (Fig. 2.27) at absolute zero of
temperature. That curve may be split into two parts with spins pointing in the
+ve z-direction and other with spin in the opposite direction, as shown in Fig.
2.27(a).
In
the absence of an external magnetic field, the distribution of electrons with
spins parallel to z-direction is equal to the number of electrons with opposite
spins and hence the net magnetic moment of the electron gas is zero.
When
a magnetic field (B) is applied along the z-direction, the energy of the spins
aligned parallel to B is lowered by the amount μg, while the energy of the
spins opposite to B is raised by the same amount (Fig. 2.27(b)).
As
a result of this, the Fermi level for the two spin distributions shift with respect
to each other and give rise to energetically unstable situation.
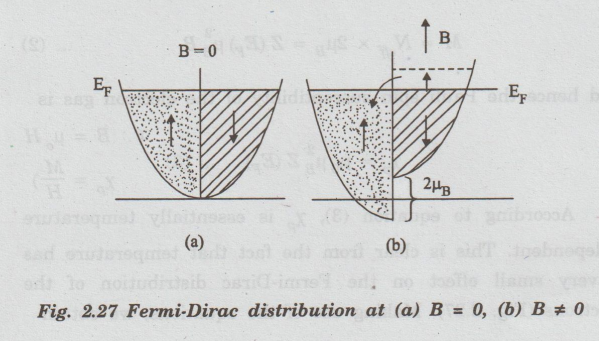
In
order to acquire the stable configuration, the electrons lying near the Fermi
level with antiparallel spins flip into the region of parallel spins until the
two Fermi levels become equal again (Fig 2.27(b)).
The
number of electrons which effectively change their direction is equal to the
density of states at the energy level (Z (E)) in one of the spin distribution
times the change in energy, i.e.
Neff
=1/2 Z (EF) µB B... (1)
where
the factor 1/2 is due to the fact that the density of states of one spin
distribution is half of the total density of states. μB is magnetic
moment of electron.
Thus
after the application of the field, the number of electrons with spins parallel
to the field is greater than the electrons with opposite spin by Neff
leading to magnetization.
Since
each flip increases the magnetization by 2μB (from –μB to
+μB), the net magnetization is given by
M
= Neff × 2μB = Z (EF) μ2B
B ...(2)
and
hence the Pauli spin susceptibility of the electron gas is
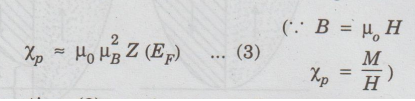
According
to equation (3), Xp is essentially temperature independent. This is clear from
the fact that temperature has a very small effect on the Fermi-Dirac distribution
of the electrons (Fig. 2.27). Making use of the equations, we obtain
Z (EF) = 3N/ 2EF
N
- No. of electrons per unit volume.
so
that (3) becomes,
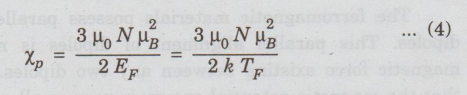
where
EF= k TF. This equation can be rewritten in terms of the
classical susceptibility as
χp
= 3/2 χ T/ TF... (5)
where
χ = µ0NµB2/kT
Since
TF is normally very high, χp is smaller than χ by about
two orders of magnitude, which is in agreement with the experimental results.
In transition metals, the paramagnetic susceptibility, χp is exceptionally
high, because Z (EF) is large.
Physics for Electrical Engineering: Unit II: b. Magnetic Properties of Materials : Tag: : Magnetic Properties of Materials - Paramagnetism in the conduction electrons in metals
Related Topics
Related Subjects
Physics for Electrical Engineering
PH3202 2nd Semester 2021 Regulation | 2nd Semester EEE Dept 2021 Regulation
