Electron Devices and Circuits: Review of Semiconductor Materials (Pre-requisite)
The Energy-Band Theory
Classification of Materials | Semiconductor Materials
• We have seen that every shell is associated with an energy level. An electron orbiting very close to the nucleus in the first shell is very much tightly bound to the nucleus and possesses only a small amount of energy.
The Energy-Band Theory
•
We have seen that every shell is associated with an energy level. An electron
orbiting very close to the nucleus in the first shell is very much tightly
bound to the nucleus and possesses only a small amount of energy. Hence first
shell has lowest energy level. Greater the distance of an electron from the
nucleus, the greater is its energy. Hence the energy level of the outermost
shell is highest. Due to such high energy, the valence electrons in the
outermost shell can be easily extracted out and hence such electrons take part
in chemical reactions and in bonding the atoms together. Now this discussion is
related to the electrons and shells of one isolated atom only.
•
In solids, atoms are brought close together. In such a case, outer shell
electrons are shared by more than one atom. So these electrons come under the
influence of forces from other atoms too. The valence electrons are shared by
forming a bond with the valence electrons of an adjacent atom. Such bonds are
called covalent bonds. Thus the valence electrons are not free under normal
conditions, as they are shared by the adjacent atoms.
•
Now the valence electrons possess highest energy level. When such electrons
form the covalent bonds, due to the coupling between the valence electrons, the
energy levels associated with the valence electrons merge into each other. This
merging forms an energy band.
•
Similarly the energy levels of various electrons present in the first orbit,
second orbit etc. also merge to form the various energy bands.
•
So instead of the presence of widely separated energy levels as that of the
isolated atoms, the closely spaced energy levels are present in a solid, which
are called energy bands.
•
Out of all the energy bands, three bands are most important to understand the
behaviour of solids. These bands are,
1.
Valence band,
2.
Conduction band,
3.
Forbidden band or gap.
Key
Point : The energy band formed due to merging of
energy levels associated with the valence electrons i.e. electrons in the last
shell, is called valence band.
•
As mentioned earlier in normal condition, valence electrons form the covalent
bonds and are not free. But when certain energy is imparted to them, they
become free.
Key
Point : The energy band formed due to merging of energy
levels associated with the free electrons is called conduction band.
•
Under normal condition, the conduction band is empty and once energy is imparted,
the valence electrons jump from valence band to conduction band and become
free.
•
While jumping from valence band to conduction band, the electrons have to cross
an energy gap.
Key
Point : The energy gap which is present separating the
conduction band and the valence band is called forbidden band or forbidden gap.
•
The energy imparted to the electrons must be greater than the energy associated
with the forbidden gap, to extract the electrons from valence band and transfer
them to conduction band. The energy associated to forbidden band is denoted as
EG.
Key
Point : The electrons cannot exist in the forbidden gap.
•
The graphical representation of the energy bands in a solid is called energy
band diagram. Such an energy band diagram for a solid silicon is shown in the
Fig. 0.3.1.
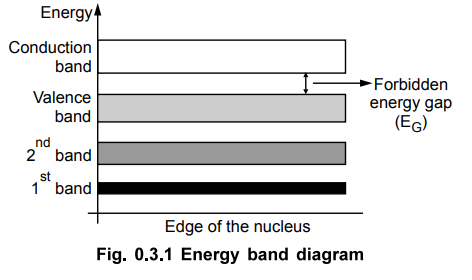
• The electrons in the various orbits
revolving around the nucleus occupy the various bands including fully or partly
occupied valence band. The conduction band which is normally empty carries the
electrons which get drifted from the valence band. These electrons present in
the conduction band are free electrons and they drift about in the spaces
between the atoms.
•
For any given type of material the forbidden energy gap may be large, small or
nonexistent. The classification of materials as insulators, conductors or
semiconductors is mainly dependent on the widths of the forbidden energy gap.
•
The energy associated with forbidden band is called energy gap EG and
measured in the unit electron-volt (eV).
1
eV = 1.6 × 10-19 J
1. Classification of Materials
•
Based on the energy gap EG, the materials are classified as
1.
Conductors 2. Insulators 3. Semiconductors.
•
In conductors, large number of free electrons exist at room temperature so EG
does not exist. The valence and conduction band are overlapped. This is shown
in the Fig. 0.3.2 (a). The examples are copper, aluminium, sliver etc.
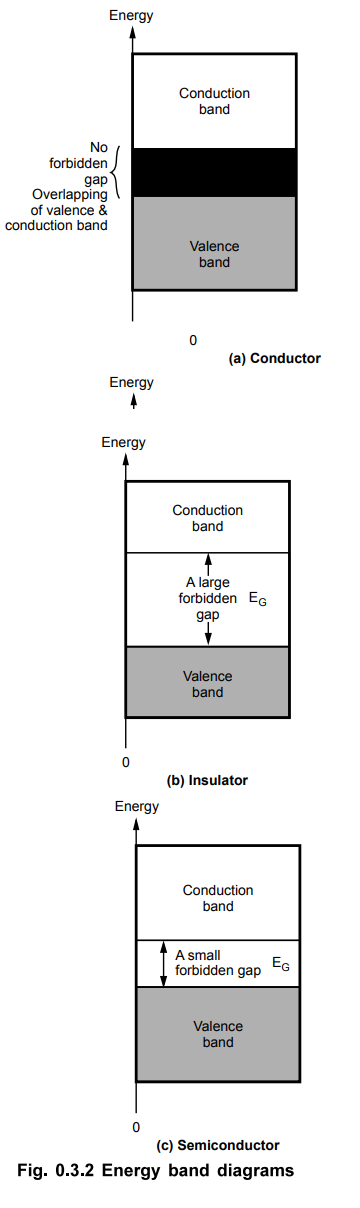
•
In insulators, the energy gap EG is large of the order of 7 eV. At very high
temperature or under high voltage also, these materials do not conduct. The
energy band diagram is shown in the Fig. 0.3.2 (b). The examples are wood, mica
paper, glass etc.
•
The semiconductors are materials in which energy gap EG is about 1
eV. At normal temperature, few free electrons exist. But at absolute zero,
these are perfect insulators. The energy gap depends on temperature. At 27 °C,
silicon has EG = 1.12 eV while germanium has EG = 0.78 eV. As
temperature increases, these materials can conduct heavily as more free
electrons are generated. The energy band diagram is shown in the Fig. 0.3.2
(c). The most important semiconductor materials are silicon (Si) and germanium
(Ge).
Electron Devices and Circuits: Review of Semiconductor Materials (Pre-requisite) : Tag: : Classification of Materials | Semiconductor Materials - The Energy-Band Theory
Related Topics
Related Subjects
Electron Devices and Circuits
EC3301 3rd Semester EEE Dept | 2021 Regulation | 3rd Semester EEE Dept 2021 Regulation
