Electron Devices and Circuits: Review of Semiconductor Materials (Pre-requisite)
The Structure of Matter
Semiconductor Materials
• The matter which occupies the space may be solid, liquid or gaseous. The molecules and atoms, of which all the substances are composed, are not at all elements but are themselves made up of simpler entities.
The Structure of Matter
•
The matter which occupies the space may be solid, liquid or gaseous. The
molecules and atoms, of which all the substances are composed, are not at all
elements but are themselves made up of simpler entities. We know this because,
we, upto certain extent, are successful in breaking atoms and studying the
resulting products. For instance, such particles of atom are obtained by causing
ultraviolet light to fall on cold metal surfaces, such particles are
spontaneously ejected from the radioactive elements. So such particles are
obtained from many different substances under widely varying conditions.
•
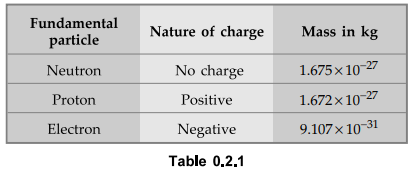
In fact, according to the modem electron theory, matter is composed of the
three fundamental particles, which are invisible to bare eyes. These are the
neutron, the proton and the electron. The proton is positively charged and the
electron is negatively charged. The neutron is uncharged i.e. electrically
neutral in nature possessing no charge. The mass of neutron and proton is same
while the electron is very light, almost l/l840th the mass of neutron. The
following table gives the information about these three particles.
•
There is no difference between an electron of copper and an electron of
aluminium or an electron of any other element. Similarly the neutrons and
protons of various atoms are characteristicwise identical in nature. Then why
do various elements behave differently ? This is because of the difference in
the arrangement of electrons, protons and neutrons of which each atom is
composed. Let us see the structure of an atom.
1. Structure of an Atom
•
The atoms have a planetary type of structure, according to classical Bohr
Model.
•
All the protons and neutrons are bound together at the centre of an atom, which
is called Nucleus. While all the electrons are moving round the nucleus. So
nucleus can be thought of as a central sun, about which electrons revolve in a
particular fashion like the planets.
•
In a normal atom the number of protons is equal to the number of electrons. As
neutron is electrically neutral, an atom as a whole is electrically neutral.
The number of protons in an atom is called as its atomic number. While the
atomic weight is approximately equal to the total number of protons and
neutrons in the nucleus of an atom.
•
The electrons which are revolving round the nucleus, do not move in the same
orbit. The electrons are arranged in the different orbits or shells at fixed
distances from the nucleus. Each shell can contain a fixed number of electrons.
In general, a shell can contain a
maximum of 2n2 electrons where n is the number of the shell. The
first shell can occupy maximum of two electrons (2 × l2) while the
second shell can occupy maximum of eight electrons (2 × 22) and so
on.
•
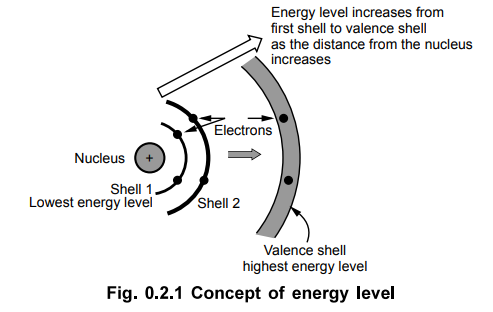
Each shell has an energy level associated with it. The closer an electron is to
the nucleus, the stronger are the forces that bind it to the nucleus. So the
first shell which is closest to the nucleus is always under the tremendous
force of attraction. While the shell which is farthest from the nucleus is
under very weak force of attraction. The electrons revolving in the last shell
i.e. farthest from the nucleus are very loosely bound to the nucleus. Such
electrons in the outermost shell are responsible for the electrical and
chemical characteristics of an atom.
Key
Point : The outermost shell is called the valence shell
and the electrons in this shell are called valence electrons.
•
The exception to the '2n2' rule stated above is that the outermost
shell in an atom cannot accommodate more than eight electrons. The valence
electrons revolving in the outermost shell are said to be having highest energy
level. The amount of energy required to extract the valence electron from the
outer shell is very less.
Key
Point : Each shell has energy level associated with it.
Closer the shell to the nucleus, more tightly it is bound to the nucleus and
possesses lower energy level.
•
Thus energy level of shell one is lowermost while the energy level of valence
shell is highest. More energy level indicates that the electrons of that shell
are loosely bound to the nucleus. Hence valence electrons are loosely bound to
the nucleus as having highest energy level. The concept of energy level is
shown in the Fig. 0.2.1.
•
When an atom absorbs energy from a heat source or from light or due to high
atmospheric temperature, the energy levels of the electrons are raised. When
such an additional energy is imparted to the electrons, the electrons move to
the next orbit which is farther from the nucleus. If such an energy is imparted
to a valence electron, it tries to jump to the next orbit. But as a valence
electron is in the outermost orbit, actually it gets completely removed from
the force of attraction of the nucleus.
Key
Point :
1)
An electron which is not subjected to the force of attraction of the nucleus is
called a free electron. Such free electrons are basically responsible to the
flow of current.
2)
More the number of free electrons, better is the conductivity of the metal.
2. Structure of Semiconductor Materials
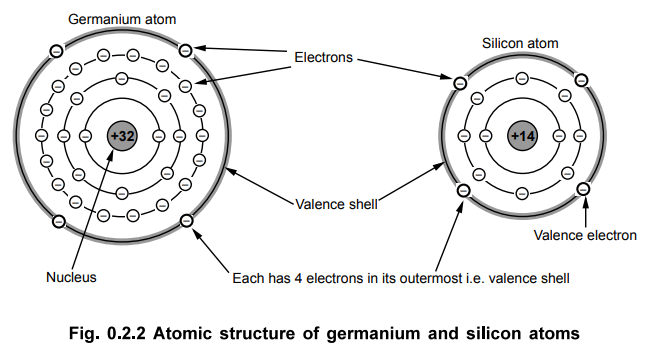
•
The semiconductor materials such as Ge and Si have four electrons in their
valence shell i.e. outermost shell. The Fig. 0.2.2 shows atomic structure of
the semiconductor materials, germanium and silicon.
•
The germanium has a nucleus with 32 protons. The electrons are distributed as
follows: 2 electrons in the first orbit, 8 in the second orbit, and 18 in the
third. The remaining four electrons are in the outer or valence orbit.
•
The silicon has nucleus with 14 protons and 14 electrons. As shown in Fig.
0.2.2 the first orbit contains 2 and the second orbit contains 8 electrons. The
remaining four electrons are in the outermost orbit.
Key
Point : When there are four electrons in the outermost
orbit, the semiconductor material is referred to as pure or intrinsic
semiconductor.
3. Ionization
•
If an electron is extracted from the outermost shell of an atom then the
overall negative charge of that atom decreases as it looses negative charge in
the form of an electron. But the number of protons remains same hence positive
charge remains same. So atom as a whole looses its electrical neutral nature
and becomes positively charged. Such an atom is called positive ion. Similarly
by any means if an electrically neutral atom gains an additional electron then
it becomes negatively charged and called negative ion. Thus by loosing or
gaining electrons, an atom gets converted into a charged ion. This process of
loosing or gaining an electron, which converts electrically neutral atom to a
charged ion is called ionization.
Electron Devices and Circuits: Review of Semiconductor Materials (Pre-requisite) : Tag: : Semiconductor Materials - The Structure of Matter
Related Topics
Related Subjects
Electron Devices and Circuits
EC3301 3rd Semester EEE Dept | 2021 Regulation | 3rd Semester EEE Dept 2021 Regulation
