Engineering Chemistry: Unit IV: b. Combustion of Fuels
Theoretical Calculation of Calorific Values
Dulong's formula with Example Solved Problems
Dulong's formula for the theoretical calculation of calorific value is GCV (or) HCV
THEORETICAL CALCULATION OF CALORIFIC VALUES
Dulong's formula
Dulong's formula for the theoretical calculation of calorific
value is
GCV (or) HCV
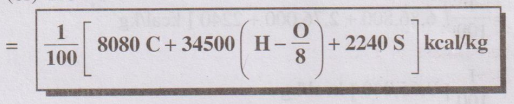
where C,H,O and S represent the % of the corresponding elements in
the fuel.
It is based on the assumption that the calorific values of C, H
and S are found to be 8080, 34500 and 2240 kcal, when 1 kg of the fuel is burnt
completely. However, all the oxygen in the fuel is assumed to be present in
combination with hydrogen in the ratio H : O as 1 : 8 by weight. So the surplus
hydrogen available for combustion is H – O/8
ஃ NCV (or) L
C V = 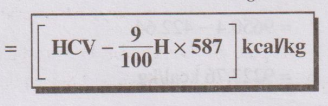
1. Problems based on calorific value
Problem 1
Calculate the gross and net calorific values of coal having the
following compositions, carbon = 85%, hydrogen = 8%, sulphur = 1%, nitrogen =
2%, ash = 4%, latent heat of steam = 587 cal/gm. (A.U. June 2007)
Solution
(i) Gross calorific value (GCV)

(ii) Net Calorific Value (NCV)

Problem 2
Calculate the gross and net calorific values of a coal sample
having the following composition C = 80% ; H = 7%; O = 3% ; S = 3.5% ; N = 2.5%
and ash 4.4%.
Solution
(i) GCV
(ii) NCV
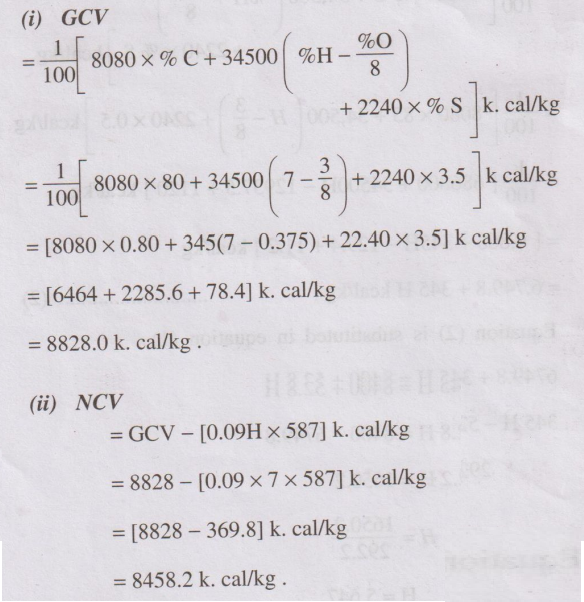
Problem 3
On analysis, a coal sample has the following compositition by
weight; C = 85%; O = 3%; S = 0.5% and
ash = 3%. Net calorific was found to be 8400 kcal/kg. Calculate the percentage
of hydrogen and gross calorific value of coal.
Solution

Problem 4
Calculate the net and gross calorific value of a coal sample
having following composition:
C = 82%, H = 8%, O = 5%, N = 1.4% and ash = 3.6%.
Solution
(i) GCV
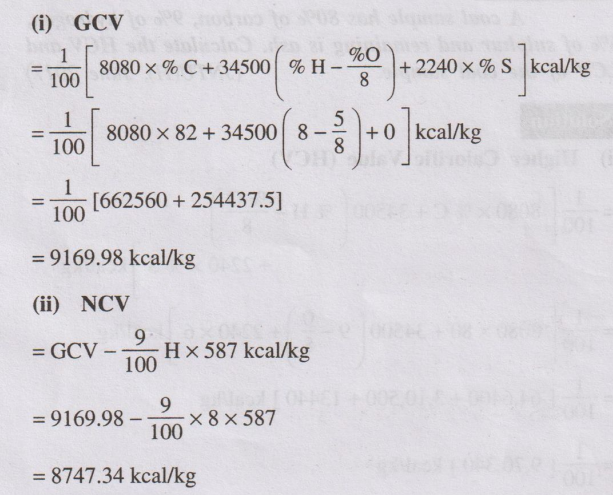
Problem 5
Calculate the gross and net calorific value of a fuel having
following composition 82% C, 8% H, 5% 0, 2.5% S, 1.4% N and 2.1% ash. (APJAKTU, July 2016)
Solution
We know that,
GCV = 1/100 [8080C + 34500 (H – O/8) + 2240S] kcal/kg
= 1/100 [8080 × 82 + 34500 (8 – 5/8) + 2240 × 2.5]
= 9225.97 kcal/kg
NCV = GCV – 0.09H × 587 kcal/kg
NCV = 9225.97 – 0.09 × 8 × 587 = 8803.3 kcal/kg
Problem 6
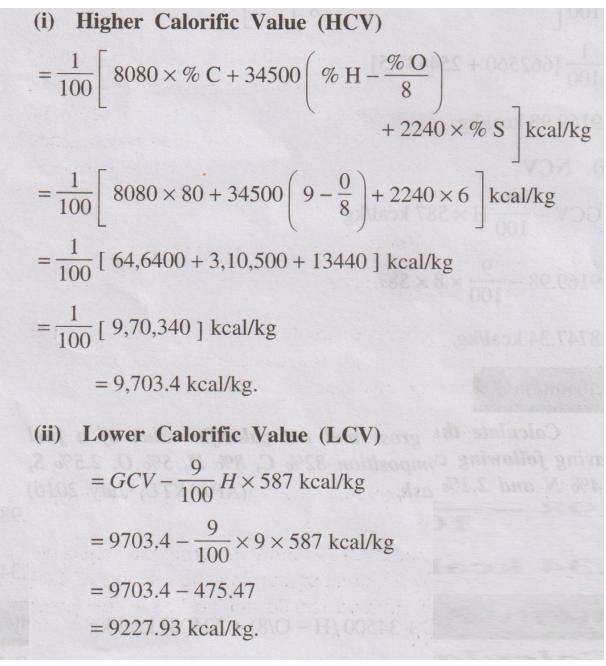
A coal sample has 80% of carbon, 9% of hydrogen, 6% of sulphur and
remaining is ash. Calculate the HCV and LCV of the coal sample. (JNTU(H), June 2017)
Solution
(i) Higher Calorific Value (HCV)
(ii) Lower Calorific Value (LCV)
Engineering Chemistry: Unit IV: b. Combustion of Fuels : Tag: Engineering Chemistry : Dulong's formula with Example Solved Problems - Theoretical Calculation of Calorific Values
Related Topics
Related Subjects
Engineering Chemistry
CY3151 1st Semester | 2021 Regulation | 1st Semester Common to all Dept 2021 Regulation
