Physics for Electrical Engineering: Unit II: a. Electrical Properties of Materials
Tight binding approximation
Electrical Properties of Materials
In solid, ionic cores at fixed lattice locations and free electron gas enveloping these ionic cores.
TIGHT
BINDING APPROXIMATION
In
solid, ionic cores at fixed lattice locations and free electron gas enveloping
these ionic cores.
In
other words, it is assumed that the solid already exists. The ionic cores are
'tightly bound' to their lattice locations. The electrons are 'free' to run through the extent of the solid. This
is called the 'Free electron
approximation'.
There
is another approach to modeling materials which starts from opposite position.
In
this approach, the atoms are independent to begin with and they are brought
together to build the solid. The electrons are bound to their respective
individual atoms to begin with.
In
this case the atoms are free to begin with while the electrons are tightly bound to the atom.
•
In view of the electronic properties of the materials, this approach is
referred to as the "Tight binding
approximation' highlighting the status of the electrons at the start of the
model.
•
Figure 2.20 shows how the tight binding
approximation builds the band structure of the solid.
•
When the atoms are far apart, all the bound electrons associated with each
atom, have fixed energy levels.
Assuming
that building the solid starts using atoms of the same element. Thus, the
energy levels occupied by the respective electrons in each atom will be
identical.
•
As we bring the atoms close to each other to form the anonibel solid, the
electrons will still maintain their original energy levels as long as the
interatomic seperation is large.
•
When the atoms get close enough, the outer shell electrons begin to overlap
with each other.
•
The energy levels of these outer shell electrons are forced to split into
energy levels above and below the energy level of these electrons when they
belong to individual atoms.
•
The splitting of energy levels occurs because electrons obey the Pauli's
exclusion principle.
•
Initially only the outer shell electrons overlap, therefore only their levels
split. But inner shell electrons still maintain their energy levels like
individual atom.
•
If the interatomic separation keeps decreasing even further, progressively more
of the inner shell electron levels will overlap and hence also split.
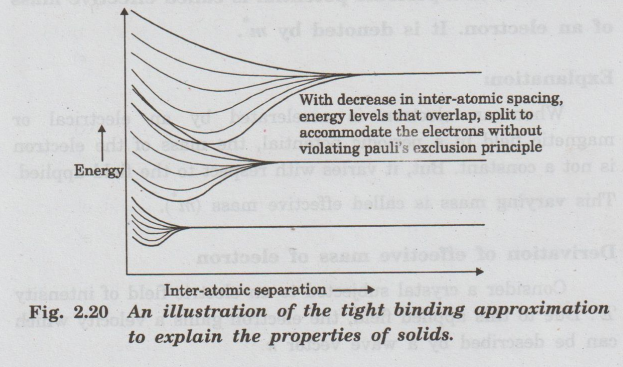
•
At each energy level, the level will split to enough new benigno energy levels
(band) so as to accommodate the electrons zinois of all the atoms in the solid
taken together.
•
For for example, if hundred atoms come together, and there is one electron in
the outer shell, the solid will split the energy level to a hundred energy
levels. Thus the hundred outer shell electrons are filled corresponding to the
combined solid.
•
In view of the starting points, the free
electron approximation lends itself more easily to the treatment of metallic
system. The tight binding approximation
is typically more consistent with the state of the material in the case of
insulators, so it is better suited for modeling insulators.
Physics for Electrical Engineering: Unit II: a. Electrical Properties of Materials : Tag: : Electrical Properties of Materials - Tight binding approximation
Related Topics
Related Subjects
Physics for Electrical Engineering
PH3202 2nd Semester 2021 Regulation | 2nd Semester EEE Dept 2021 Regulation
