Physics for Electrical Engineering: Unit V: Nano Devices
Bandgap of nanomaterials
Nano Devices
The electronic properties of metals and semiconductors are determined by their electronic band structure. The band structure changes with particle size.
BANDGAP OF NANOMATERIALS
The
electronic properties of metals and semiconductors are determined by their
electronic band structure. The band structure changes with particle size.
Molecular orbitals get converted into delocalized band states as shown in fig.
5.8.
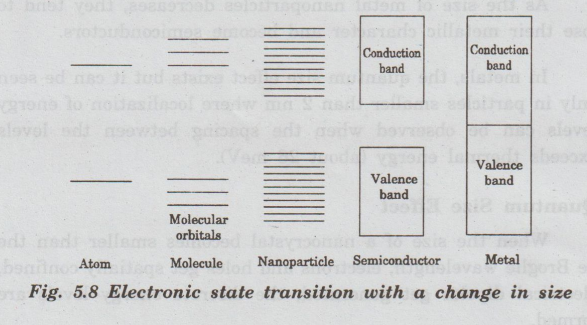
The band structure of nanocrystals lies between the discrete density of states as in atoms and molecules and continuous band as in crystals.
As
the size of the material decreases, the energy separation between the adjacent
levels increases. This size quantization effect is responsible for the
transition of electronic states from a bulk metal or semiconductor to
nanoparticles.
The
particles that show this size quantization effect are called Q particles or
quantum dots.
In
case of the particle size being less than the de Broglie wavelength, charge
carriers can be quantum-mechanically understood as particles in a box and the
size of the box can provide the dimensions of the particle.
With
a decrease in particle size of metals, the quasi-continuous density of states
splits into discrete electronic levels with an increase in the spacing between
these levels.
Quantum
size effect is most significant for semiconductor nanoparticles. In
semiconductor, a bandgap already exists in the bulk state. It also increases
and the energy bands gradually convert into discrete molecular electronic
levels with a decrease in particle size.
As
the size of metal nanoparticles decreases, they tend to lose their metallic
character and become semiconductors.
In
metals, the quantum size effect exists but it can be seen only in particles
smaller than 2 nm where localization of energy levels can be observed when the
spacing between the levels exceeds thermal energy (about 26 meV).
Quantum
Size Effect
When
the size of a nanocrystal becomes smaller than the de Broglie wavelength,
electrons and holes get spatially confined, electrical dipoles get generated,
the discrete energy levels are formed.
As
the size of the material decreases, the energy separation between adjacent
levels increases. The density of states of nanocrystals is positioned in
between discrete (as that of atoms and molecules) and continuous (as in
crystals). я ИО ущіл
Quantum
size effect is most significant for semiconductor nanoparticles. In
semiconductors, the bandgap energy is of the order of a few electron volts. It
increases with a decrease in particle size.
When
photons of light fall on a semiconductor, the photons are absorbed. A sudden
rise in absorption is observed when the photon energy is equal to the bandgap.
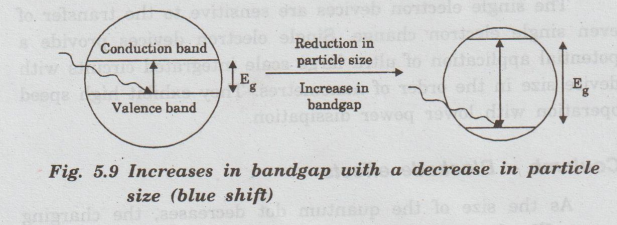
As
the size of the particle decreases, absorption shifts towards the shorter
wavelength (blue shifts). This indicates an increase in the bandgap energy
(Fig.5.9). A change in absorption causes a change in the colour of the
semiconductor nanoparticle.
For
example, bulk cadmium sulfide is orange in colour and has a bandgap of 2.42 eV.
It becomes yellow and then ultimately white as its particle size decreases and
the bandgap increases.
TUNNELING
The
phenomenon of penetration of charge carriers directly through the potential
barrier, instead of climbing over it, is called tunneling.
Physics for Electrical Engineering: Unit V: Nano Devices : Tag: : Nano Devices - Bandgap of nanomaterials
Related Topics
Related Subjects
Physics for Electrical Engineering
PH3202 2nd Semester 2021 Regulation | 2nd Semester EEE Dept 2021 Regulation
