Physics for Electrical Engineering: Unit I: Dielectric Materials and Insulation
Electronic Polarisation
Calculation | Polarization mechanisms in dielectrics
Electronic polarisation is due to the displacement of positively - charged nucleus and negatively – charged electrons of an atom in opposite directions on the application of an electrical field. This induces dipole moment in the dielectric.
ELECTRONIC
POLARISATION
Electronic
polarisation is due to the displacement of positively - charged nucleus and
negatively – charged electrons of an atom in opposite directions on the
application of an electrical field. This induces dipole moment in the
dielectric.
Dipole
moment (μ) is proportional to the electric field strength (E).
i.e.,
μ ∝ Ε
μ
= αe Ε
where
αe is proportionality constant and it is known as electronic
polarisability.
Calculation of Electronic
polarisability (αe)
(i) Without electric field
Consider
an atom of a dielectric material of nuclear charge + Ze at the centre (point
change), where Z is the atomic number. The electrons of charge (- Ze) are
distributed uniformly throughout the atom (sphere) of radius R as shown in fig.
1.4.

The
centres of electron cloud and positive nucleus are at the same point and hence
there is no dipole moment.
Negative
charge density of atom is given by
ρ
= Total negative charge / Volume of the atom = - Z e / 4/3 πR3
ρ
= -3/4 Ze / πR3 .........(1)
(ii) With electric field
When
the atom of the dielectric is placed in an electrical field of strength E, two
phenomena occur
(a)
Lorentz force (due to electrical field) tends to move the nucleus and electron
cloud of that atom from their equilibrium positions.
The
positive nucleus moves towards the field direction and the electron cloud moves
in opposite direction of the field as shown in fig.1.5.

(b)
After separation, an attractive Coulomb force arises between the nucleus and
the electron cloud which tends to maintain the original equilibrium position.
When
these two forces are equal and opposite, there is a new equilibrium between the
nucleus and electron cloud of the atom.
The
electron cloud and the nucleus are separated by a distance 'x'. It results in
formation of electrical dipole in the atom.
Lorentz
force between nucleus and electron FL = Charge × electrical field
=
ZeE... (2)
Coulomb
attractive force (FC) between nucleus and electron cloud being
separated at a distance x,
FC
= Qp Qe / 4πε0 x2 ................(3)
=
Nuclear plaib Charge (Ze) × Total negative charges enclosed in the sphere of
radius x / 4πε0x2
Total
negative charges enclosed in the sphere of radius x = Charge density (ρ) × Volume
of the sphere of radius x
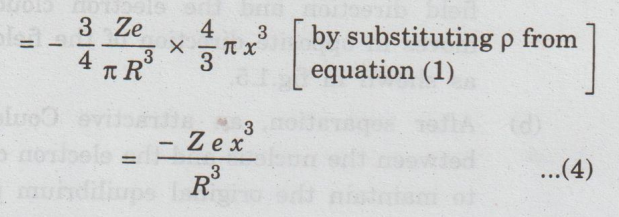
Total
positive charge of atom present in the sphere of radius x,
Qp
= + Ze (being a point charge)
Substituting
the equation (4) in (3), we have
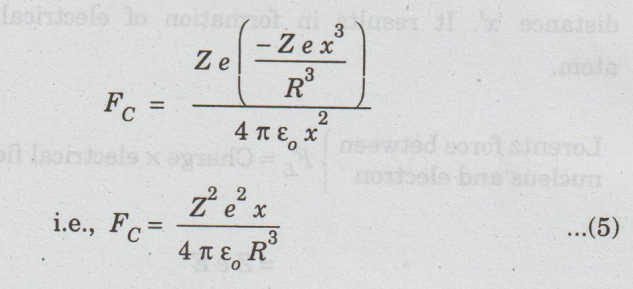
At
equilibrium, Coulomb force and Lorentz force must be equal and opposite.
i.e.,
FL=- - FC
Substituting
for FL and FC from equation (2) and (5), we have
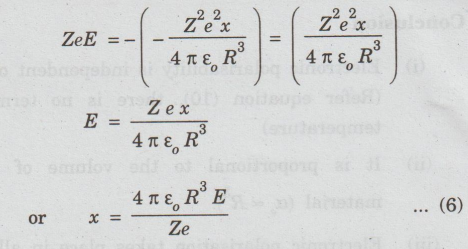
Due
to the application of electrical field on the atom, the charge centres are
displaced from their equilibrium position and hence the atom gains some dipole
moment.
From
the definition of dipole moment, induced dipole moment (μind) is
given by
μind
= Magnitude of charge × Displacement
i.e.,
μind = Z e x........(7)
Substituting
equation (6) in (7), we have
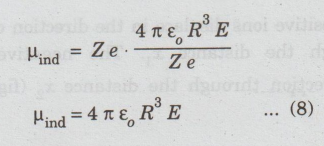
But,
the induced dipole moment
in
terms of polarisability is given by,
μind
= ɑe E.............(9)
where
ɑe is called as
electronic polarisability.
On
comparing the equations (8) and (9), we have
αe
= 4 πε0 R3.................(10)
Conclusion
(i)
Electronic polarisability is independent of temperature. (Refer equation (10),
there is no term representing temperature)
(ii)
It is proportional to the volume of atoms in the material (αe - R3).
(iii)
Electronic polarisation takes place in all dielectrics.
Physics for Electrical Engineering: Unit I: Dielectric Materials and Insulation : Tag: : Calculation | Polarization mechanisms in dielectrics - Electronic Polarisation
Related Topics
Related Subjects
Physics for Electrical Engineering
PH3202 2nd Semester 2021 Regulation | 2nd Semester EEE Dept 2021 Regulation
